ExploringCeftolozane's Revolutionary R&D Successes and its Mechanism of Action on Drug Target
Ceftolozane's R&D Progress
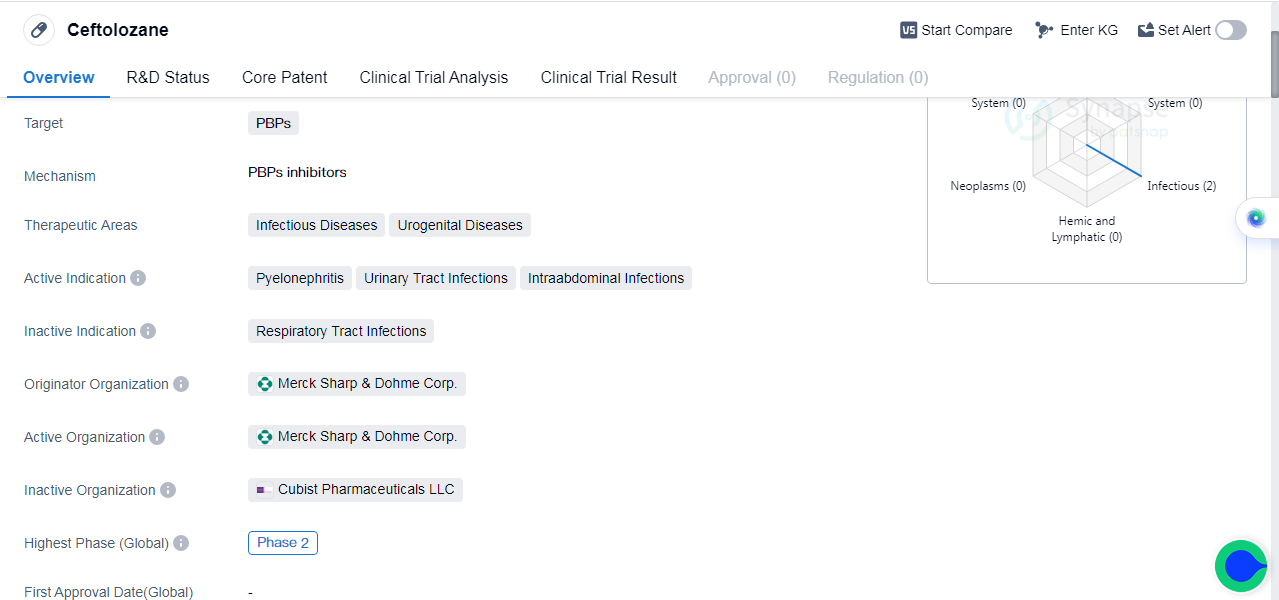
Ceftolozane is a small molecule drug that falls under the category of biomedicine. It primarily targets PBPs (Penicillin-Binding Proteins) and is used in the treatment of pyelonephritis, urinary tract infections, and intraabdominal infections.
Ceftolozane was developed by Merck Sharp & Dohme Corp., a renowned pharmaceutical organization. Currently, Ceftolozane is in its highest phase of development, which is phase 2.
In terms of therapeutic areas, Ceftolozane is primarily focused on treating infectious diseases and urogenital diseases. Infectious diseases refer to illnesses caused by pathogenic microorganisms such as bacteria, viruses, fungi, or parasites. On the other hand, Urogenital diseases are conditions that affect the urinary and reproductive systems.
Pyelonephritis is a type of urinary tract infection that affects the kidneys. Urinary tract infections (UTIs) are infections that occur in any part of the urinary system, including the bladder, urethra, and kidneys. Intraabdominal infections refer to infections that occur within the abdominal cavity, which can be caused by various factors such as surgery, trauma, or underlying medical conditions.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Ceftolozane: PBPs inhibitors
PBPs inhibitors are a type of medication that target and inhibit the activity of penicillin-binding proteins (PBPs). PBPs are enzymes found in bacterial cell walls that play a crucial role in the synthesis and maintenance of the cell wall structure. By inhibiting PBPs, these drugs prevent the cross-linking of peptidoglycan chains, which weakens the bacterial cell wall and ultimately leads to cell lysis and death.
From a biomedical perspective, PBPs inhibitors are important in the field of antimicrobial therapy. They are commonly used to treat bacterial infections caused by susceptible organisms. By targeting PBPs, these inhibitors can effectively disrupt the integrity of bacterial cell walls, make them an effective strategy to combat bacterial pathogens. Different classes of PBPs inhibitors exist, such as beta-lactam antibiotics and glycopeptides, each with their own mechanisms of action and spectrum of activity.
It's worth noting that PBPs inhibitors primarily target bacterial PBPs, they are not effective against human PBPs. This selectivity allows these drugs to specifically target bacterial cells while minimizing harm to human cells. However, the emergence of antibiotic resistance in bacteria poses a challenge to the effectiveness of PBPs inhibitors, as bacteria can develop mechanisms to evade the inhibitory effects of these drugs.
Drug Target R&D Trends for Ceftolozane
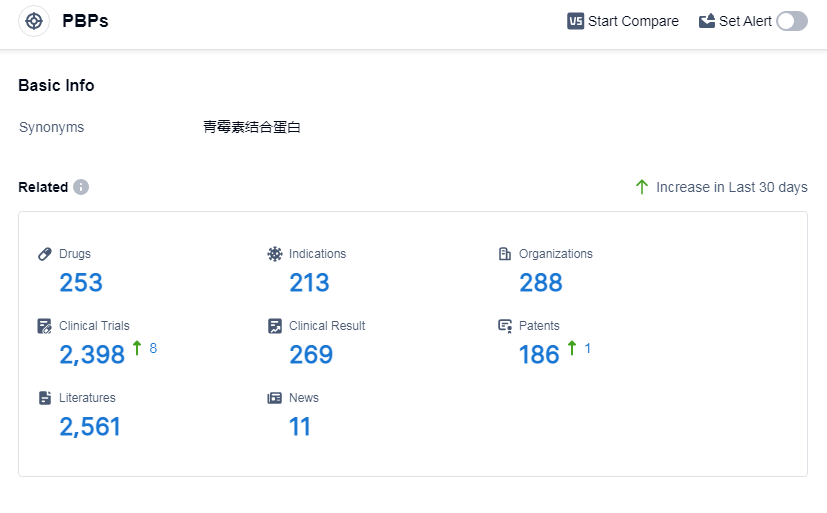
According to Patsnap Synapse, as of 5 Sep 2023, there are a total of 253 PBPs drugs worldwide, from 288 organizations, covering 213 indications, and conducting 2398 clinical trials.
Pfizer Inc. is a leading company in the development of drugs targeting PBPs, with a significant number of drugs in various stages of development.The most common indications for drugs under the target PBPs include bacterial infections, infectious diseases, and urinary tract infections. Small molecule drugs and synthetic peptides are the drug types progressing most rapidly, indicating potential competition with biosimilars. China leads the way in terms of approved drugs under the target PBPs. Overall, the current competitive landscape for target PBPs is dynamic, with active R&D efforts and potential for future development in various countries and companies.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Ceftolozane is a small molecule drug developed by Merck Sharp & Dohme Corp. It targets PBPs and is primarily used in the treatment of infectious diseases and urogenital diseases. Ceftolozane shows promise in the treatment of pyelonephritis, urinary tract infections, and intraabdominal infections. As it progresses through phase 2 of development, further evaluation will determine Ceftolozane's potential efficacy and safety for broader use in the pharmaceutical industry.