Deep Scientific Insights on Tesofensine's R&D Progress, Mechanism of Action, and Drug Target
Tesofensine's R&D Progress
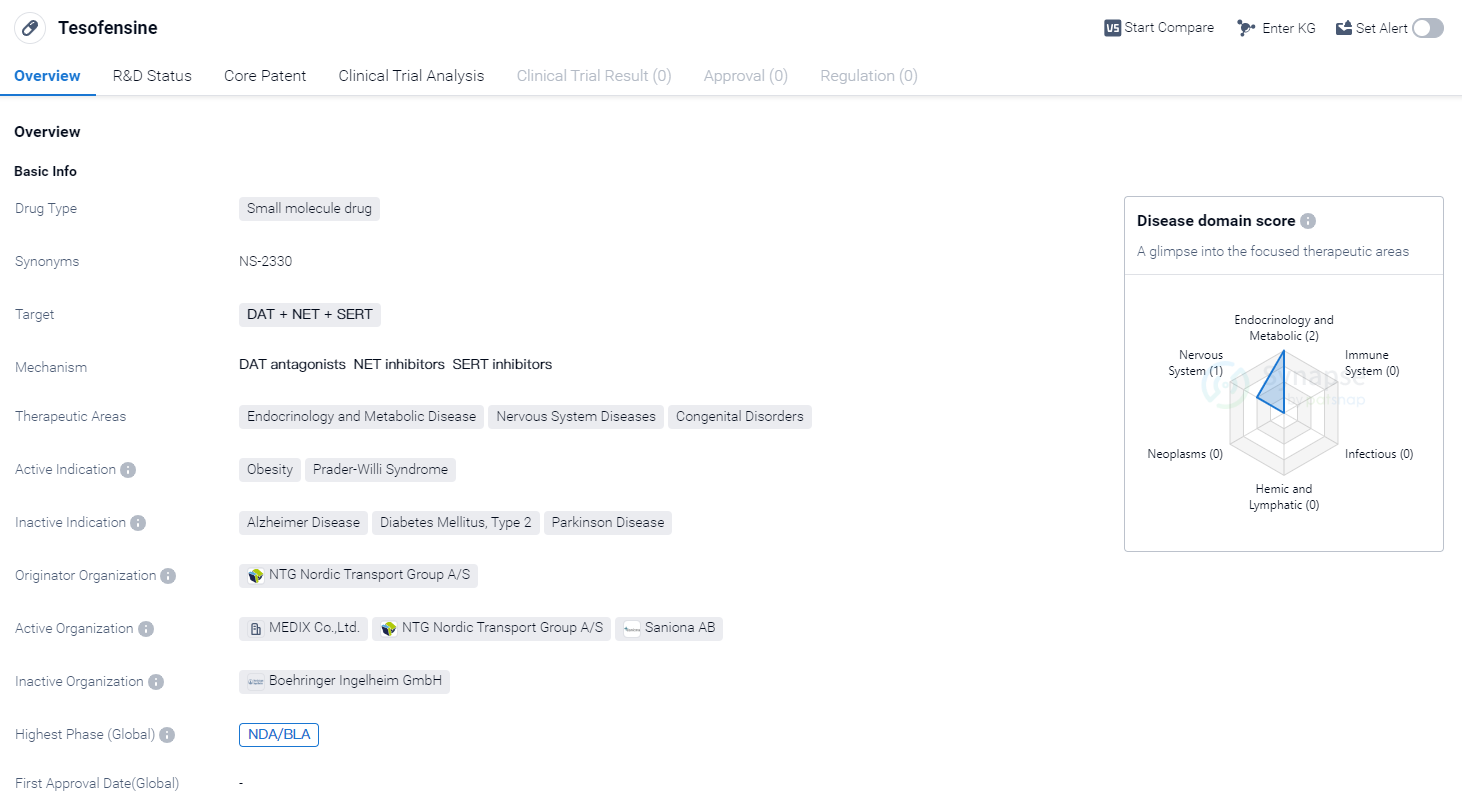
Tesofensine is a small molecule drug that targets DAT (dopamine transporter), NET (norepinephrine transporter), and SERT (serotonin transporter). It is primarily used in the treatment of obesity and Prader-Willi Syndrome. The drug falls under the therapeutic areas of endocrinology and metabolic disease, nervous system diseases, and congenital disorders.
Tesofensine is developed by NTG Nordic Transport Group A/S, an originator organization in the pharmaceutical industry. The drug has reached the highest phase of development, which is NDA/BLA (New Drug Application/Biologics License Application).
Obesity is a global health concern, and there is a significant need for effective treatments. Tesofensine's mechanism of action, targeting DAT, NET, and SERT, suggests that it may help regulate the neurotransmitters dopamine, norepinephrine, and serotonin, which play a role in appetite control and metabolism. By modulating these neurotransmitters, Tesofensine may help reduce food cravings and increase satiety, leading to weight loss.
Prader-Willi Syndrome is a rare genetic disorder characterized by excessive hunger, leading to obesity and other health complications. Tesofensine's potential use in this indication suggests that it may help address the underlying appetite dysregulation seen in individuals with Prader-Willi Syndrome.
The development of Tesofensine by NTG Nordic Transport Group A/S highlights the company's commitment to addressing unmet medical needs in the field of biomedicine. The drug's progression to the NDA/BLA phase indicates promising results from preclinical and clinical studies, and the company's intention to bring this innovative therapy to market.
Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Tesofensine: DAT antagonists, NET inhibitors, and SERT inhibitors
DAT antagonists, NET inhibitors, and SERT inhibitors are terms related to biomedicine and pharmacology.
1. DAT antagonists: DAT stands for Dopamine Transporter. DAT antagonists are drugs or compounds that block the function of the dopamine transporter, which is responsible for reuptake of dopamine from the synapse back into the presynaptic neuron. By inhibiting the reuptake of dopamine, DAT antagonists increase the concentration of dopamine in the synapse, leading to increased dopamine signaling and neurotransmission. These drugs are commonly used in the treatment of conditions such as attention deficit hyperactivity disorder (ADHD) and narcolepsy.
2. NET inhibitors: NET stands for Norepinephrine Transporter. NET inhibitors are drugs that block the function of the norepinephrine transporter, which is responsible for reuptake of norepinephrine from the synapse back into the presynaptic neuron. By inhibiting the reuptake of norepinephrine, NET inhibitors increase the concentration of norepinephrine in the synapse, leading to increased norepinephrine signaling and neurotransmission. These drugs are used in the treatment of various conditions, including depression, attention disorders, and certain types of pain.
3. SERT inhibitors: SERT stands for Serotonin Transporter. SERT inhibitors are drugs that block the function of the serotonin transporter, which is responsible for reuptake of serotonin from the synapse back into the presynaptic neuron. By inhibiting the reuptake of serotonin, SERT inhibitors increase the concentration of serotonin in the synapse, leading to increased serotonin signaling and neurotransmission. These drugs are commonly used in the treatment of depression, anxiety disorders, and obsessive-compulsive disorder (OCD).
Overall, DAT antagonists, NET inhibitors, and SERT inhibitors are different classes of drugs that act on specific transporters in the brain to modulate the levels of dopamine, norepinephrine, and serotonin, respectively.
Drug Target R&D Trends for Tesofensine
According to Patsnap Synapse, as of 5 Sep 2023, there are a total of 27 DAT + NET + SERT drugs worldwide, from 46 organizations, covering 33 indications, and conducting 198 clinical trials.
The analysis of the current competitive landscape and future development of target DAT + NET + SERT reveals that Luye Pharma Group Ltd. is the leading company with the highest stage of development. The R&D progress of various companies indicates a strong focus on developing drugs targeting DAT + NET + SERT. Several drugs have been approved for indications such as Obesity, Depressive Disorder, and Attention Deficit Disorder With Hyperactivity. Small molecule drugs are progressing rapidly, indicating potential advancements in this drug type. China, along with other countries like the United States, European Union, and Japan, is developing rapidly in this target, with significant progress in drug development. Overall, the target DAT + NET + SERT shows promise in addressing various therapeutic areas and has a competitive landscape with potential for future growth and innovation.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In conclusion, Tesofensine is a small molecule drug that targets DAT, NET, and SERT and is being developed by NTG Nordic Transport Group A/S. It shows potential in the treatment of obesity and Prader-Willi Syndrome, addressing unmet medical needs in the areas of endocrinology and metabolic disease, nervous system diseases, and congenital disorders. The drug has reached the highest phase of development, indicating its advanced stage of testing and potential for regulatory approval.