Extracellular Vesicles Give the Pharmaceutical Industry A Shot in The Arm
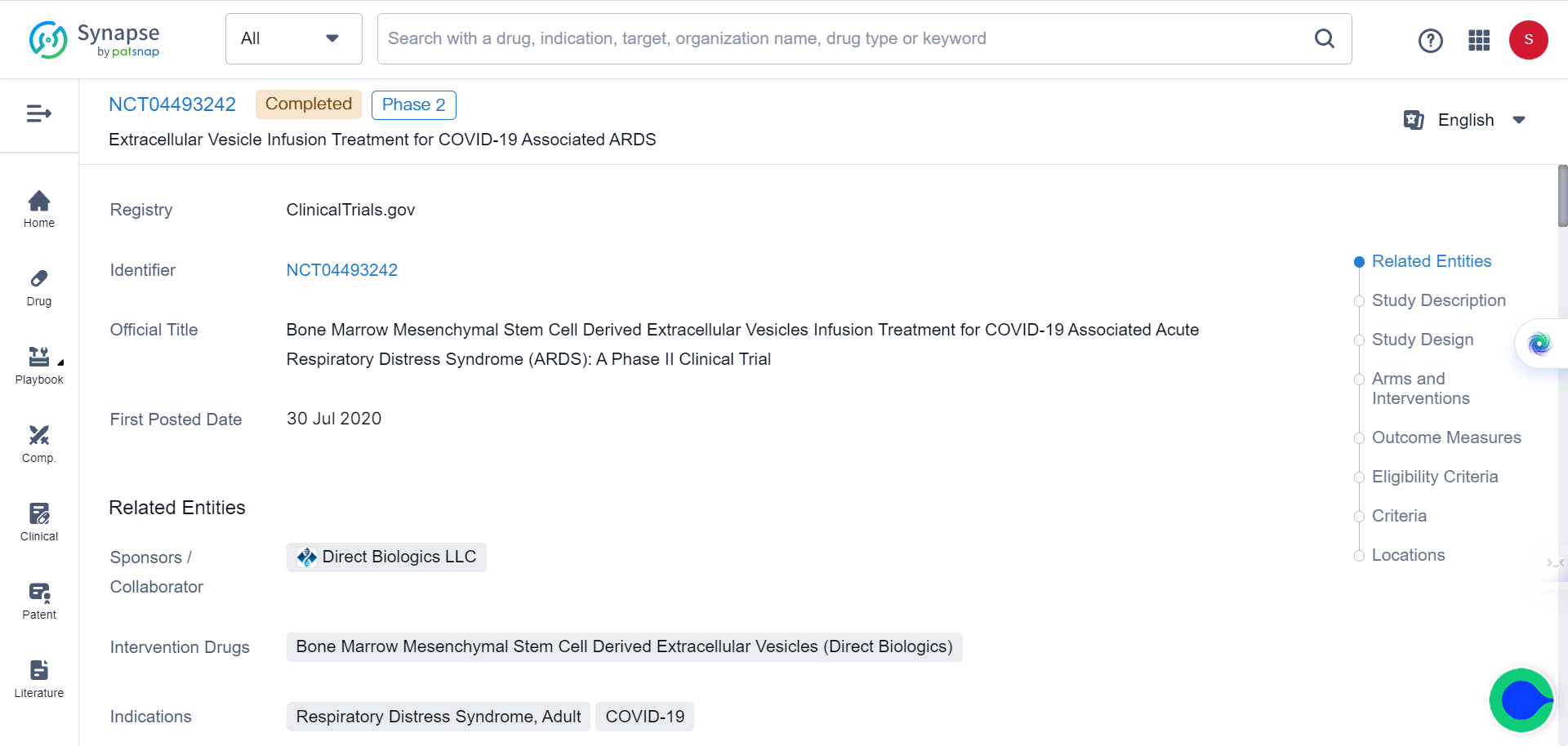
On July 12, Direct Biologics has announced positive safety and efficacy results from its Phase 2 clinical trial (NCT04493242) of ExoFlo in adult COVID-19 patients with moderate-to-severe acute respiratory distress syndrome (ARDS).
EVs for COVID
The Texas-based biotech company has conducted a randomized, double-blind, placebo-controlled clinical trial to investigate the efficacy and safety of extracellular vesicles (EVs) derived from bone marrow mesenchymal stem cells (BM-MSCs) in treating respiratory failure caused by severe COVID-19. The study, named Extracellular Vesicle Infusion Treatment for COVID-19 (EXIT COVID-19), was conducted at five sites across the United States and involved 102 patients.
The researchers aimed to determine whether two doses of ExoFlo, the EV product manufactured by Direct Biologics, would safely reduce mortality in patients with moderate-to-severe acute respiratory distress syndrome (ARDS) caused by COVID-19, compared to a placebo. The primary outcome measure was the all-cause 60-day mortality rate, while secondary outcomes included time to death, incidence of treatment-emergent serious adverse events, proportion of discharged patients at 7, 30, and 60 days, time to hospital discharge, and ventilation-free days.
The results of the study showed that ExoFlo was safe in treating patients with severe or critical COVID-19 respiratory failure. Mortality (60-day) in the intention-to-treat (ITT) population was reduced in ExoFlo-15 (15 mL dose) compared to the placebo group, although the difference was not significant. However, a post-hoc subgroup analysis showed that the risk reduction in 60-day mortality was significant in ExoFlo-15 compared to the placebo group in participants aged 18 to 65 with moderate-to-severe ARDS. Ventilation-free days were also significantly improved in this subgroup.
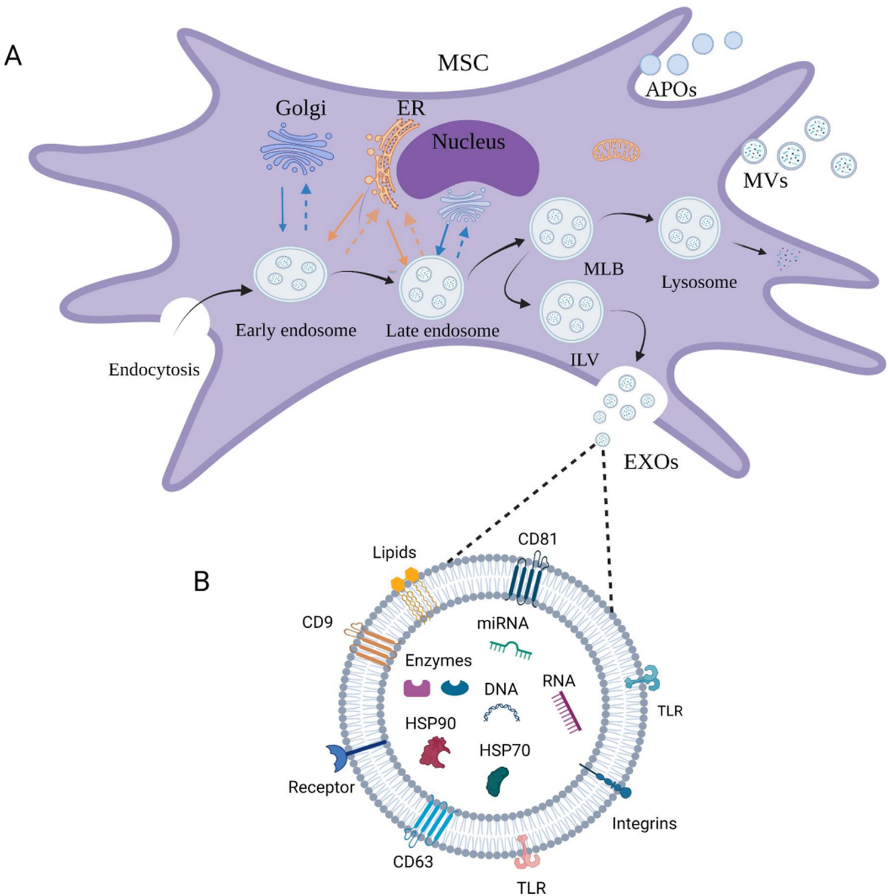
ExoFlo is an acellular product manufactured per current good manufacturing practice (CGMP) regulations from a single donor BM-MSC culture that conveys the immunomodulatory and regenerative properties of BM-MSC without cellular therapy limitations. The EVs in ExoFlo are fully characterized to meet the International Society for Cell and Gene Therapy (ISCT) definition of possessing trilineage differentiation capability (bone, adipose, and cartilage) and to be positive for the surface markers CD90 and CD166 but negative for CD45. The cells are evaluated by, and have a master file on record, with the FDA that includes information about the chemistry, manufacturing, and controls (CMC) requirements for an approved Phase II IND clinical study.
Safe ride with EVs
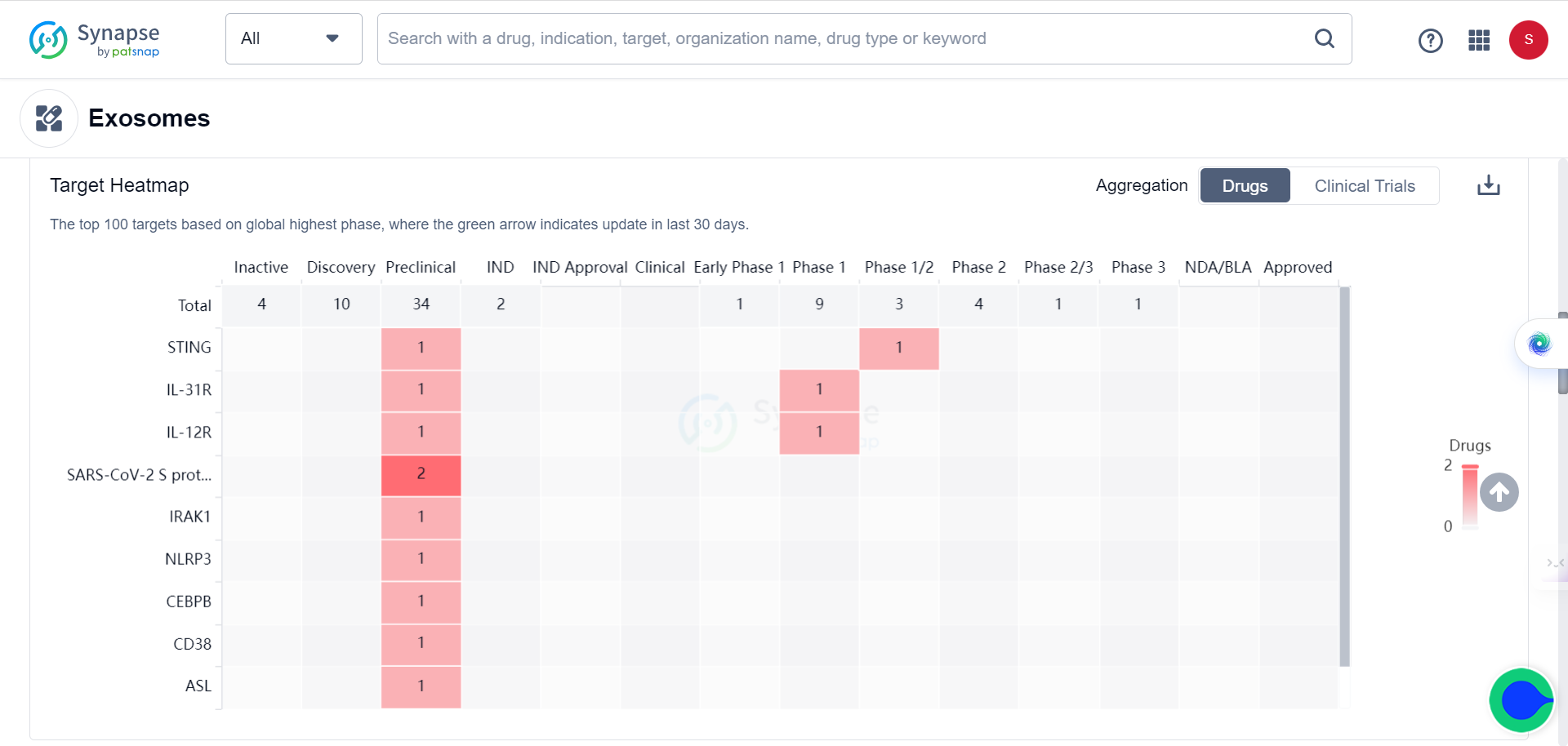
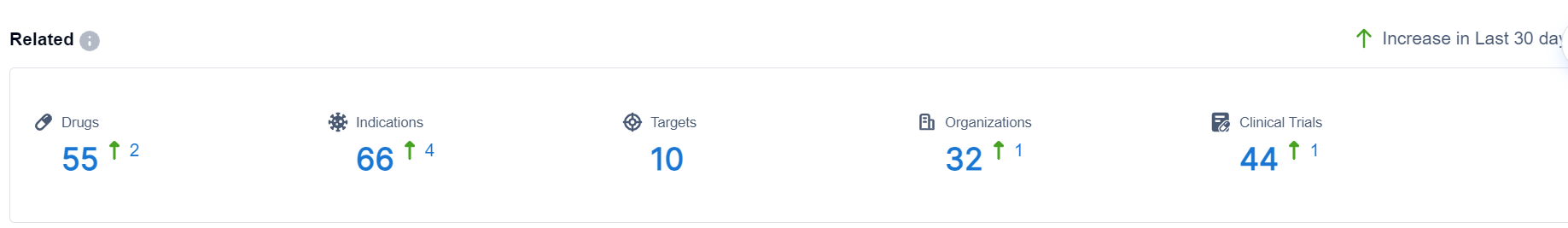
This achievement represents the current trend of utilizing EVs as a delivery system for various medications, including vaccines, antibiotics, and anti-cancer drugs. According to the Synapse database, there are currently 55 drugs from 32 companies in various stages of development. Additionally, new players are entering the field as evidenced by one organization and two drugs that emerged within the last 30 days.
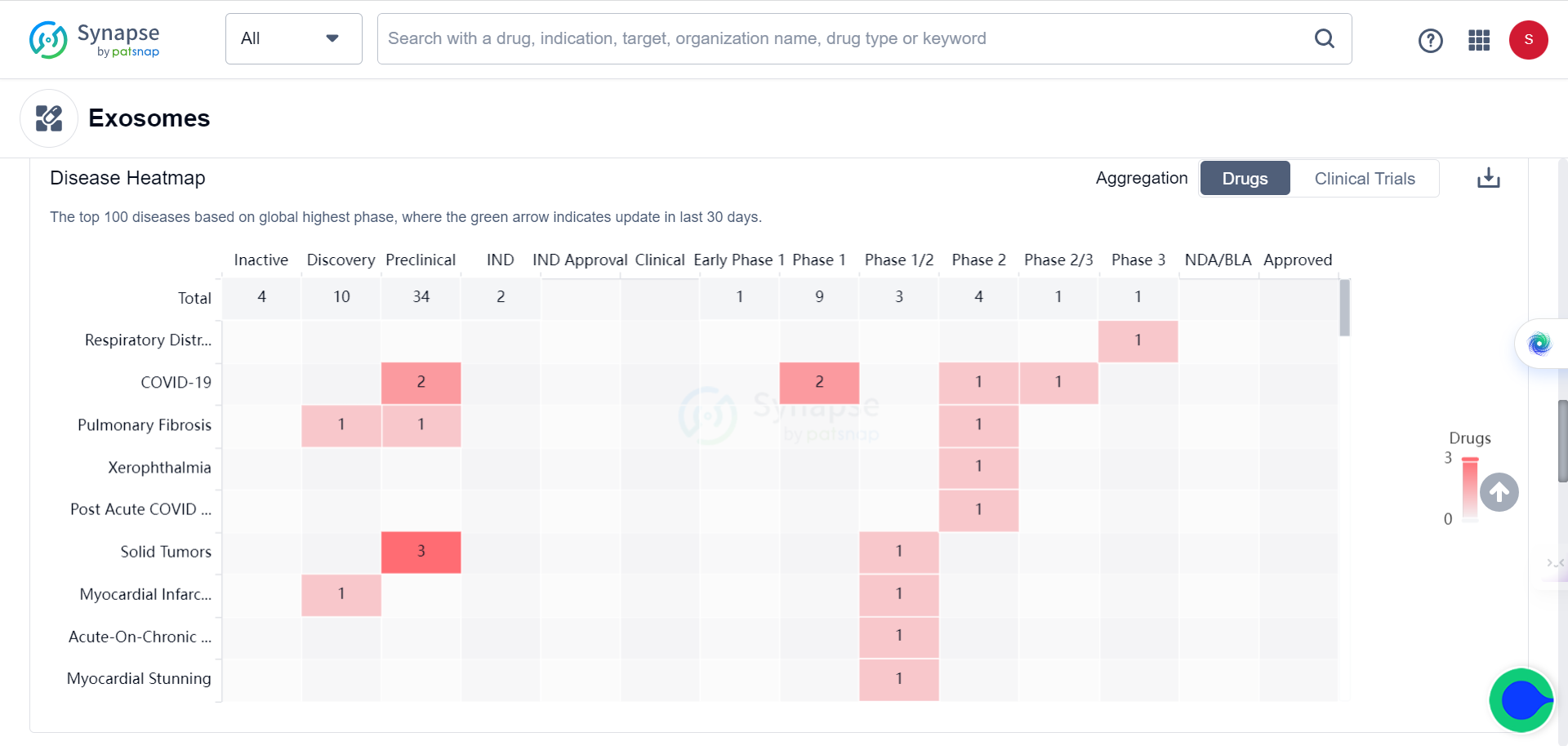
The list of targets and indications keep growing, from initially for treating respiratory diseases and COVID to solid tumors.
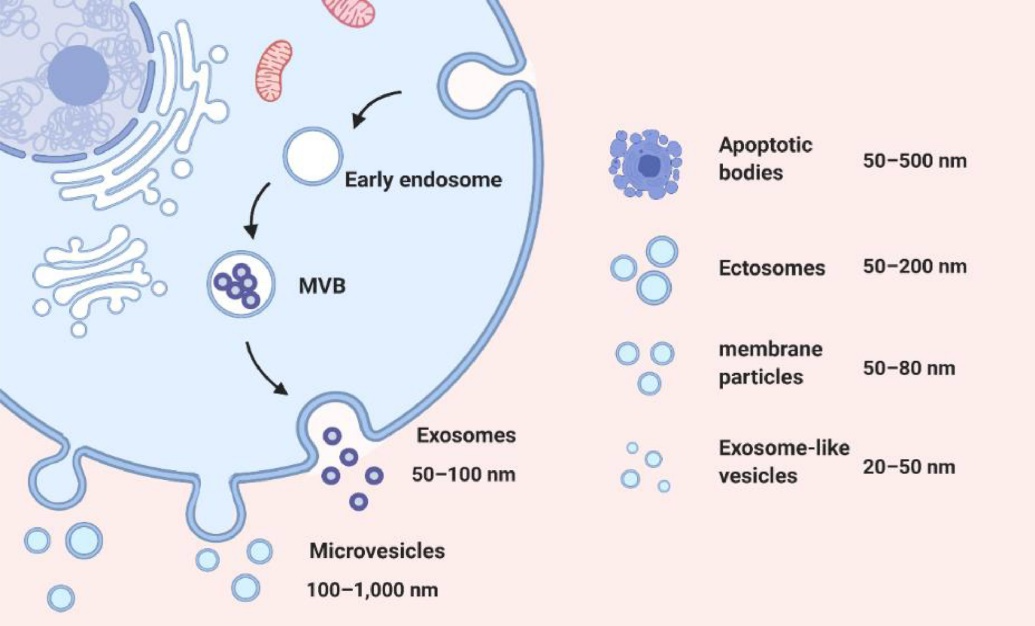
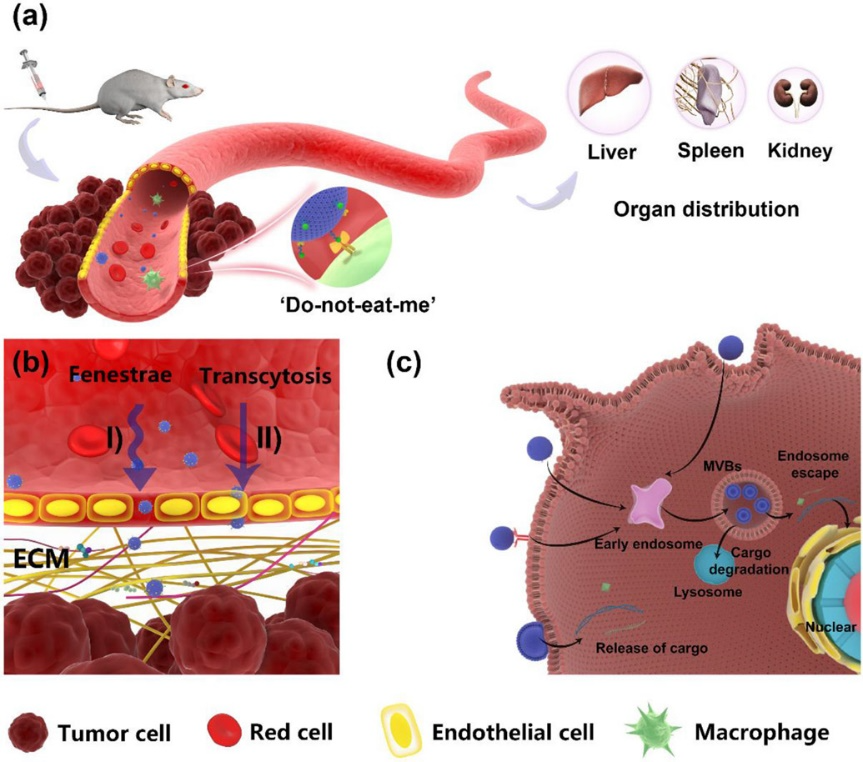
EVs show great promise as delivery systems for drugs and imaging agents due to their unique characteristic, such as size, surface charge, and lipid composition, allowing them to protect cargo from degradation and cross biological barriers like the blood-brain barrier. Their mechanical properties enable them to fuse with cell membranes and self-replicate, making them efficient carriers for therapeutic and diagnostic cargo.
In addition, EVs contain endogenous biomolecules like proteins, nucleic acids, and lipids, giving them a more natural and biocompatible profile compared to synthetic nanoparticles, reducing the risk of toxicity and immunogenicity.
However, some challenges remain. EVs exhibit considerable heterogeneity that can impact their biological activities and properties. The limited capacity to carry large therapeutic payloads also restrict EVs’ use in delivering some agents. And the methods for isolating EVs from tissue/cell cultures or biological samples can be time-consuming, expensive and require specialized equipment.
To address these challenges, researchers are developing customized engineering of EVs and new cargo-loading approaches, as well as improvements in separation techniques. Continued research and innovation are needed to create an advanced drug delivery system.
Powerhouse alliance
EVs have the potential to be combined with other trendy new technologies to create an even greater impact. Evox Therapeutics, a UK-based biotech company, has recently acquired exosome adeno-associated virus (AAV) technology from a US-based biotech company, Takeda. This acquisition will allow Evox to enhance its exosome-based drug delivery platform.
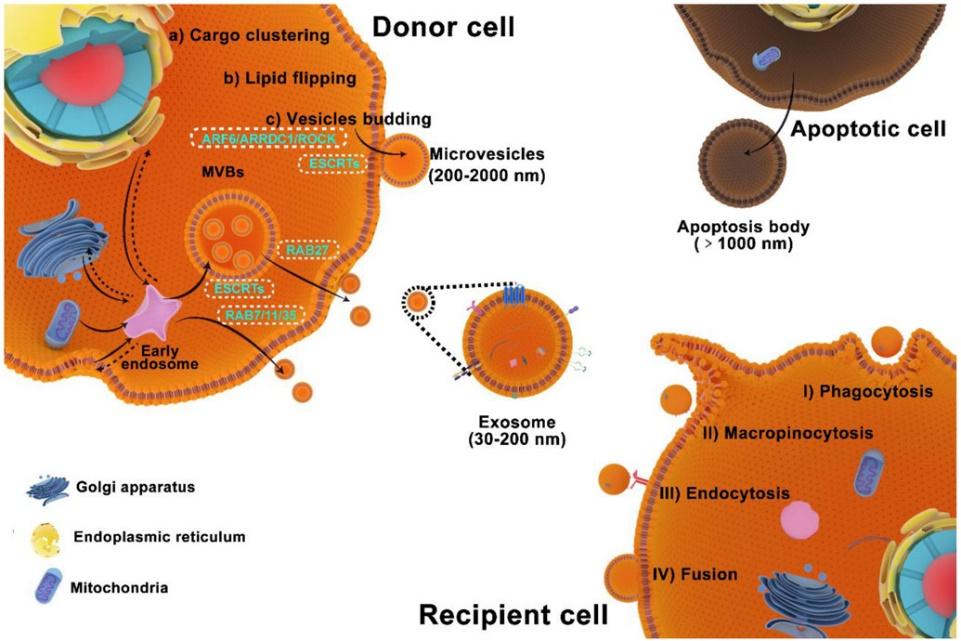
Exosomes, a subpopulation of EVs, are small vesicles produced naturally by cells that play a role in intercellular communication. Due to their ability to cross biological barriers and target specific cells, exosomes have the potential to be used as drug delivery vehicles.
AAVs, on the other hand, are a type of virus commonly used in gene therapy to deliver therapeutic genes to cells. The combination of exosomes and AAVs can improve the delivery of therapeutic genes to target cells.
Evox intends to use the exosome AAV technology to develop treatments for rare diseases and cancer. The company's pipeline already includes EVX-101, an exosome-based therapy for Niemann-Pick disease type C, currently in phase 1 clinical trials. With the acquisition of exosome AAV technology, Evox can strengthen its drug delivery platform and develop more effective therapies for a variety of diseases.
This is just another example that the advent of EVs will transform the landscape of drug delivery and give the pharmaceutical industry a shot in the arm.

References:
1.Lightner AL, Sengupta V, Qian S, Ransom JT, Suzuki S, Park DJ, Melson TI, Williams BP, Walsh JJ, Awili M, Bone Marrow Mesenchymal Stem Cell Derived Extracellular Vesicle Infusion for the Treatment of Respiratory Failure from COVID-19: A Randomized Placebo Controlled Dosing Clinical Trial, CHEST (2023).
2.Fu P, Zhang J, Li H, Mak M, Xu W, Tao Z. Extracellular vesicles as delivery systems at nano-/micro-scale. Adv Drug Deliv Rev. 2021 Dec;179:113910.