Vorolanib - Betta's New Multitargeted Drug for Kidney Cancer Treatment
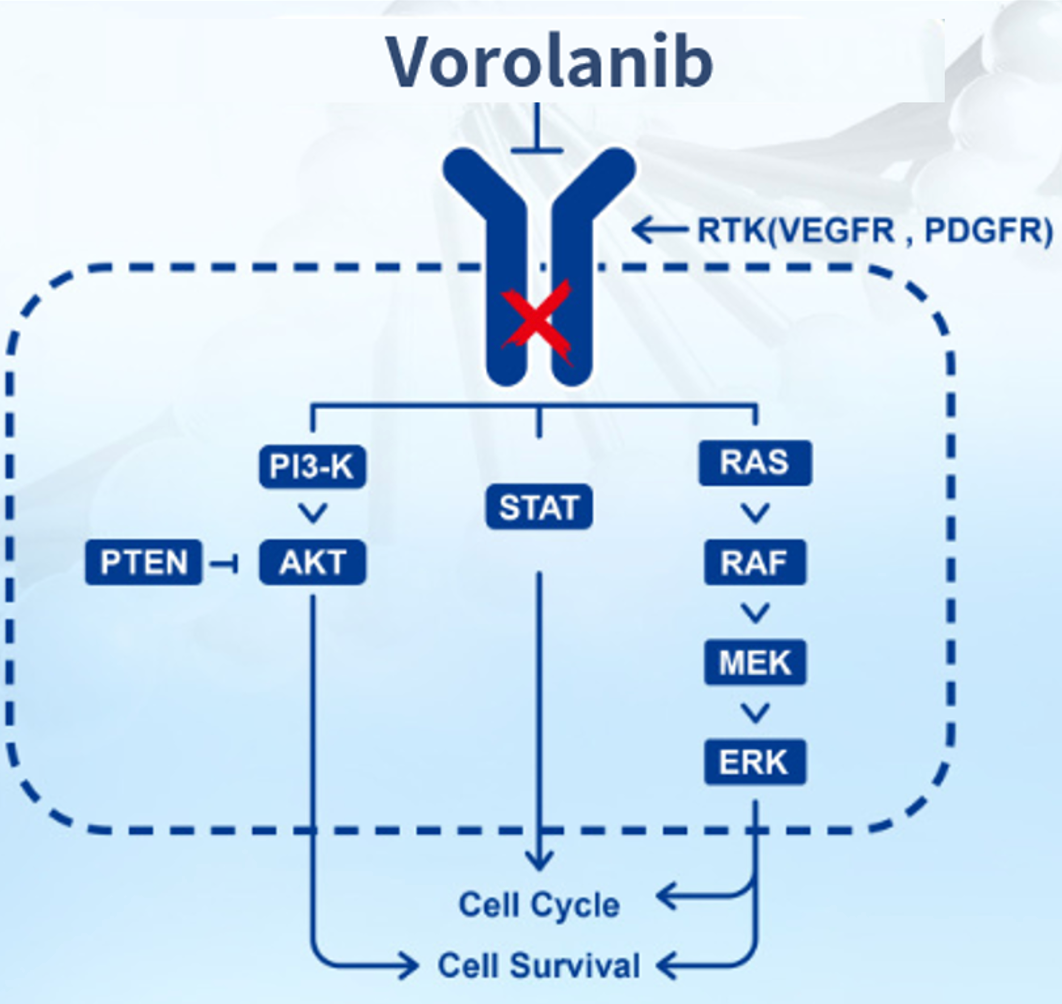
Vorolanib, is a small molecule drug targeting PDGFR+VEGFR (see Figure 2-1 for molecular structure). This product was originally developed by Tyrogenex, Inc., with the main inventors being Liang Congxin, Gao Shu, and Li Zhigang. In 2017, Betta Pharmaceuticals Co., Ltd. acquired rights for all indications of Vorolanib in China. On June 7, 2023, the China National Medical Products Administration (NMPA) approved Vorolanib. This drug is used in combination with Everolimus for patients with advanced renal cell carcinoma who have previously failed to receive tyrosine kinase inhibitor treatment.
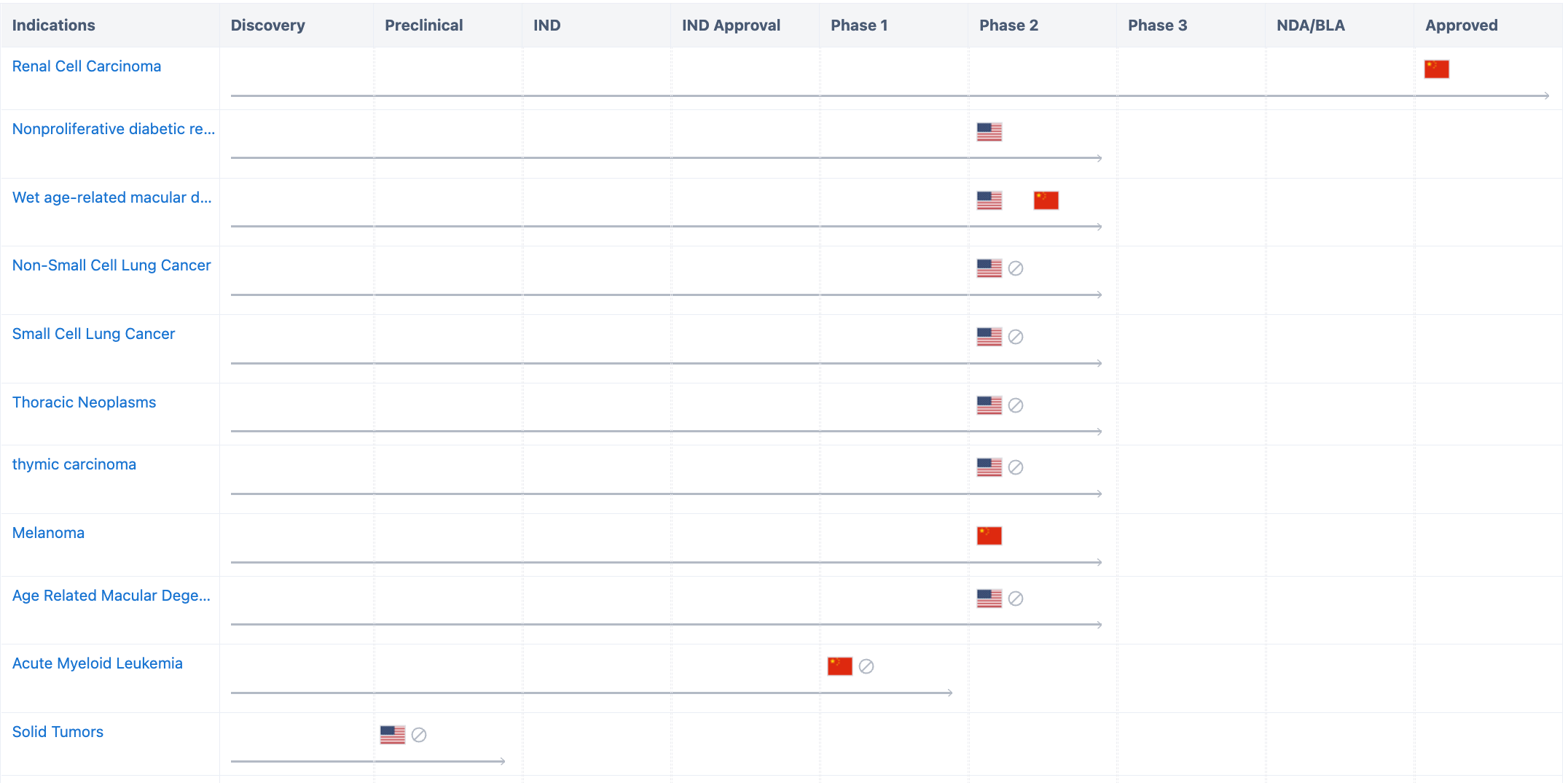
According to “Synapse”, the drug involves a total of 12 indications, including renal cell carcinoma, nonproliferative diabetic retinopathy, wet age-related macular degeneration, melanoma, diabetic macular oedema, etc. The majority of indications are currently in the clinical trial phase, with research primarily being conducted in China and the United States. Alongside the approved indication renal cell carcinoma, swift progress has also been made in researching various ophthalmic diseases, which have now entered the clinical phase 2. For more detailed information on R&D status, core patents, analysis, etc., please click on the image link below.
Vorolanib is a new generation of multi target kinase inhibitors with a completely new chemical structure.This is a multi-target receptor tyrosine kinase inhibitor with potent inhibitory effects on VEGFR2, KIT, PDGFR, FLT3, and RET. Its main anti-tumor mechanism involves the inhibition of angiogenesis.
Clinical Trial Results
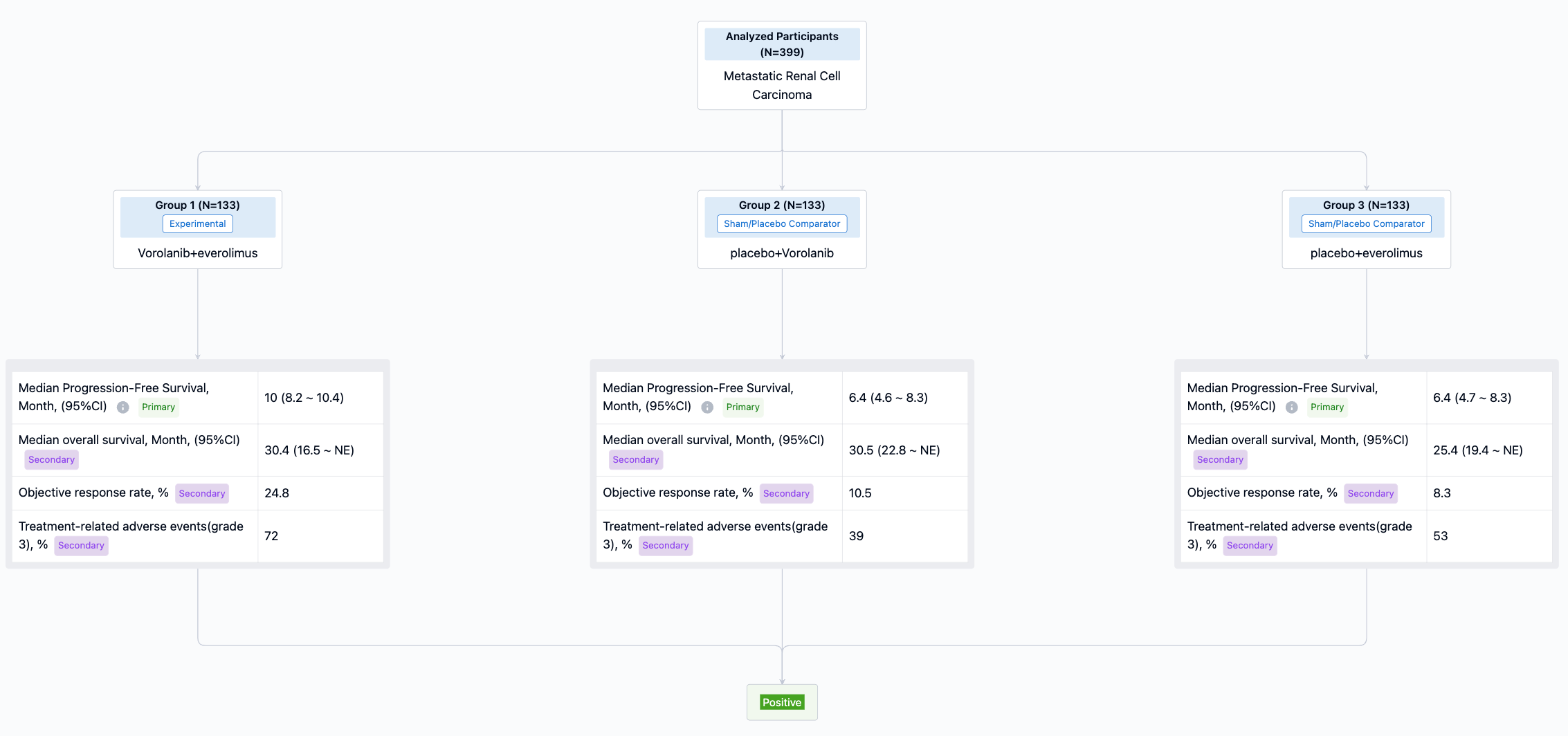
A randomized, phase 3, double-blind, multicenter trial evaluated the efficacy and safety of Vorolanib, everolimus, and the combination in patients with pretreated metastatic renal cell carcinoma (CONCEPT study, NCT03095040) . Results showed: The median PFS in combination group was significantly longer than that in single-agent everolimus group (10.0 months vs. 6.4 months; P = 0.0171), while the median PFS was similar between single-agent vorolanib group and everolimus group (6.4 months vs. 6.4 months). OS was immature with no significant difference between pts assigned vorolanib plus everolimus (30.4 months) and those allocated everolimus (25.4 months ) or vorolanib (30.5 months).
Treatment-related adverse event (TRAE) occurred in 132 (99%) of 133 pts with vorolanib plus everolimus, 127 (96%) of 133 pts with single-agent vorolanib and 131 (99%) of 133 pts with single-agent everolimus, respectively. Grade 3 or higher TRAEs occurred in fewer patients who received single-agent vorolanib (52 [39%]) than in those who received single-agent everolimus (71 [53%]) or vorolanib plus everolimus (96 [72%]). Safety profiles of both agents were consistent with previous studies.
Competitive Landscape
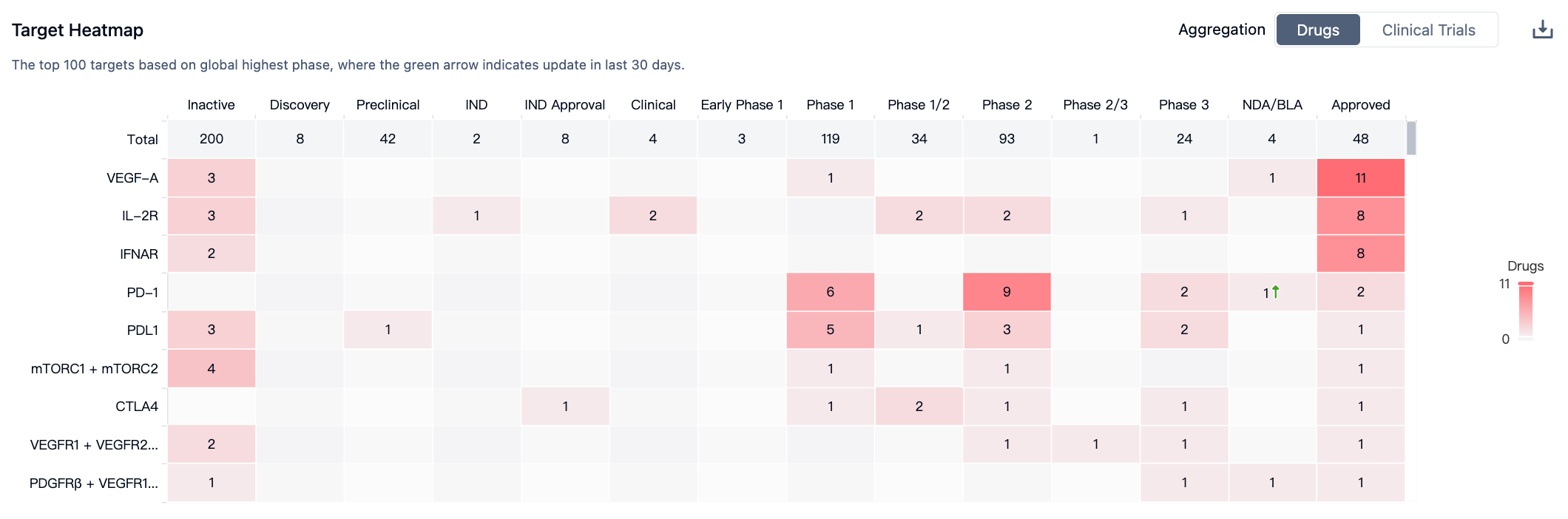
According to “Synapse”, there are a total of 520 competing drugs with the same indications for renal cell carcinoma, of which 200 drugs are already in "Inactive" state, 48 drugs have been approved, and the target of approved drugs is focused on VEGF-A. For more information, please click on the image link below.