EZHARMIA® Approved in Japan as First Dual EZH1/EZH2 Inhibitor for Peripheral T-Cell Lymphoma Therapy
Japan has granted approval for Daiichi Sankyo’s EZHARMIA® (valemetostat tosilate) to be used in treating adult patients with relapsed or refractory peripheral T-cell lymphoma. EZHARMIA stands as the first dual inhibitor of EZH1 and EZH2 to be endorsed for PTCL, following its SAKIGAKE designation for this purpose.
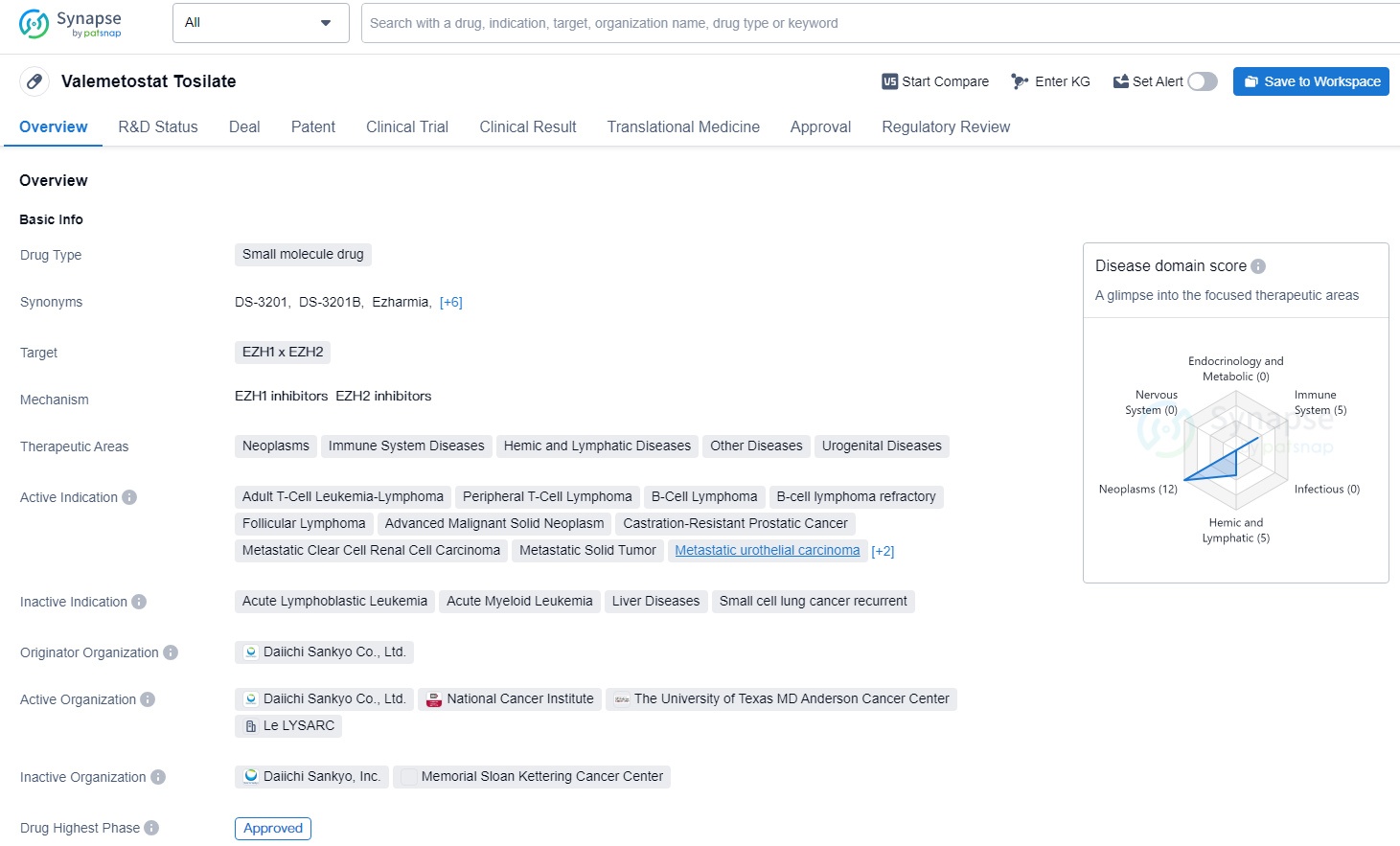
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
PTCL is a category of uncommon and frequently aggressive blood cancers, making up roughly 10 to 15% of all non-Hodgkin lymphomas. PTCL has a higher prevalence in Asian regions, such as Japan, compared to other global areas. Most individuals diagnosed with PTCL experience disease advancement after the initial treatment with a multi-drug chemotherapy regimen, with the median overall survival following a relapse standing at about 5.8 months.
The endorsement of EZHARMIA by Japan's Ministry of Health, Labour and Welfare is rooted in findings from the VALENTINE-PTCL01 phase 2 trial, presented at the 2023 American Society of Hematology Annual Meeting.
“This second approval for EZHARMIA in Japan marks a significant step forward in treating relapsed or refractory peripheral T-cell lymphoma, as there is an urgent need for new and effective treatment options to enhance patient outcomes,” stated Toshinori Agatsuma, PhD, Executive Officer, Head of R&D Division in Japan, Daiichi Sankyo. “EZHARMIA represents the cutting-edge research undertaken by Daiichi Sankyo to develop new drugs that can potentially redefine the standard of care for cancer patients.”
The safety profile of EZHARMIA in the VALENTINE-PTCL01 trial matched that observed in previous clinical studies. Treatment-related adverse events were noted in 106 out of 133 patients, with the most frequent being platelet count reduction, anemia, dysgeusia, and neutrophil count reduction.
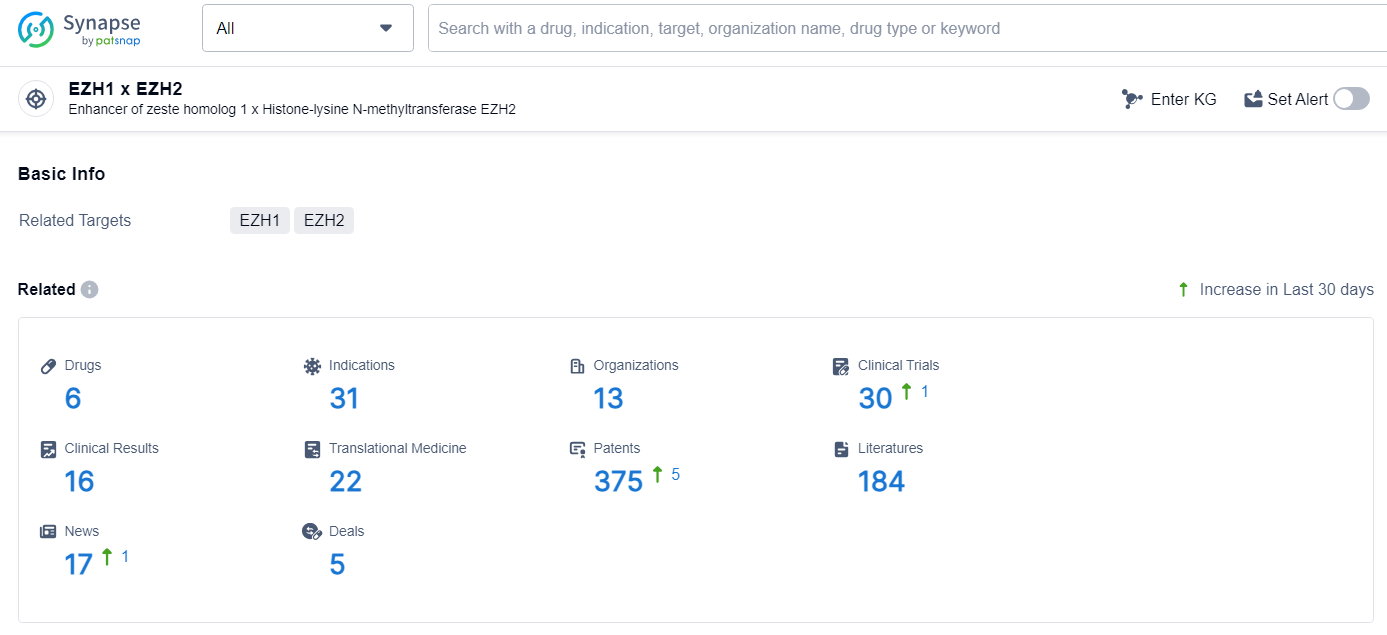
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of July 1, 2024, there are 6 investigational drugs for the EZH1 and EZH2 target, including 31 indications, 13 R&D institutions involved, with related clinical trials reaching 30, and as many as 375 patents.
Valemetostat Tosilate is a small molecule drug with a broad range of therapeutic indications, particularly in the treatment of various types of cancer. Its approval in Japan in 2022 marks an important milestone in the pharmaceutical industry, offering potential new treatment options for patients with these specific indications. As an approved orphan drug, Valemetostat Tosilate may provide hope for patients with rare diseases or conditions, and its development represents a significant advancement in the field of biomedicine.