Fasenra Approved in the US for Eosinophilic Granulomatosis with Polyangiitis Treatment
AstraZeneca’s Fasenra (benralizumab) received approval in the United States for treating adult individuals diagnosed with eosinophilic granulomatosis with polyangiitis (EGPA). EGPA is an uncommon, immune-mediated inflammation of blood vessels that can lead to damage in various organs and may be deadly if not treated.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The US Food and Drug Administration (FDA) granted approval based on encouraging findings from the MANDARA Phase III trial, which were published in The New England Journal of Medicine. This study evaluated the safety and effectiveness of Fasenra compared to the only approved treatment for EGPA, mepolizumab, in individuals with relapsing or refractory EGPA. Notably, MANDARA was the first head-to-head non-inferiority trial of biologics for EGPA patients. Participants were randomized to receive either a 30 mg subcutaneous injection of Fasenra or three 100 mg subcutaneous injections of mepolizumab every four weeks.
In the study, nearly 60% of patients treated with Fasenra achieved remission, a result similar to that seen in patients treated with mepolizumab. Moreover, data indicated that 41% of Fasenra-treated patients successfully weaned off oral corticosteroids (OCS), compared to 26% in the mepolizumab group (difference: 16%; 95% CI: 1,31).
Dr. Michael Wechsler, a Professor of Medicine and Director of The Asthma Institute at National Jewish Health, and the International Coordinating Investigator for the MANDARA trial, commented: “This approval is highly encouraging for US patients with EGPA who are burdened by severe symptoms. Patients frequently depend on long-term oral corticosteroids, which carry significant and lasting side effects. Benralizumab represents a crucial treatment option, demonstrating that remission is within reach for EGPA sufferers, and also aiding in reducing steroid use.”
Joyce Kullman, the Executive Director of the Vasculitis Foundation, stated: “This disease severely affects patients and their quality of life, necessitating more treatment alternatives. The approval of another EGPA treatment brings hope to approximately 15,000 patients in the US facing this challenging rare disease.”
Ruud Dobber, Executive Vice President of AstraZeneca's BioPharmaceuticals Business Unit, remarked: “Fasenra is already established for severe eosinophilic asthma treatment, and with this approval, US physicians can offer a new, convenient monthly subcutaneous injection to EGPA patients. Today's announcement highlights Fasenra's potential in assisting patients with eosinophilic diseases beyond severe asthma.”
The safety and tolerability profile of Fasenra in the MANDARA trial aligned with its known profile.
Around half of EGPA patients have adult-onset severe eosinophilic asthma (SEA) and often exhibit sinus and nasal symptoms. Fasenra is only the second biologic authorized for this disease's treatment.
Currently, Fasenra is approved as an add-on maintenance therapy for SEA in over 80 countries, including the US, Japan, the EU, and China. Furthermore, it is approved for children and adolescents aged six and older in the US and Japan. The FDA designated Fasenra as an Orphan Drug for EGPA in 2018.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
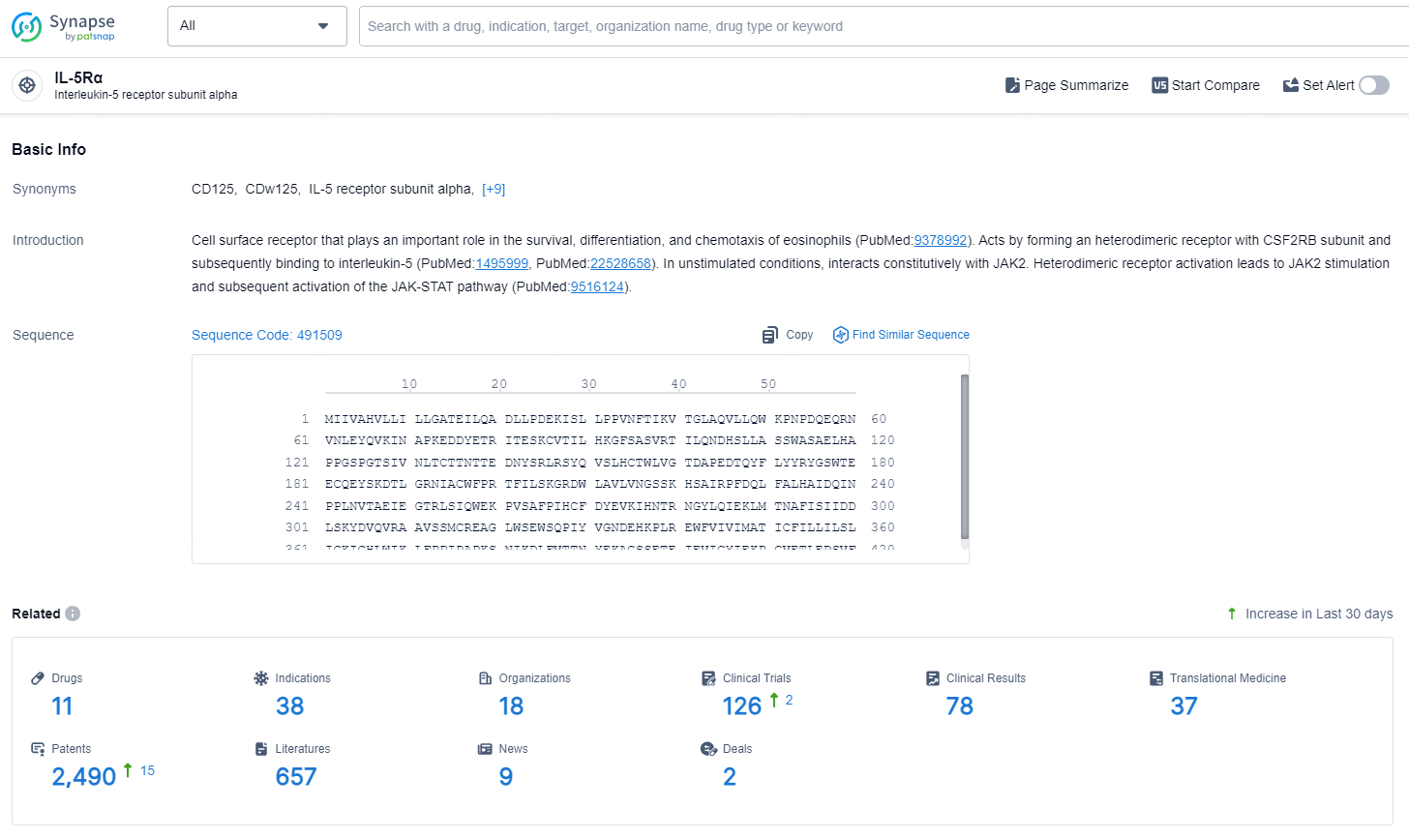
According to the data provided by the Synapse Database, As of September 18, 2024, there are 32 investigational drugs for the IL-5Rα targets, including 26 indications, 31 R&D institutions involved, with related clinical trials reaching 20, and as many as 6241 patents.
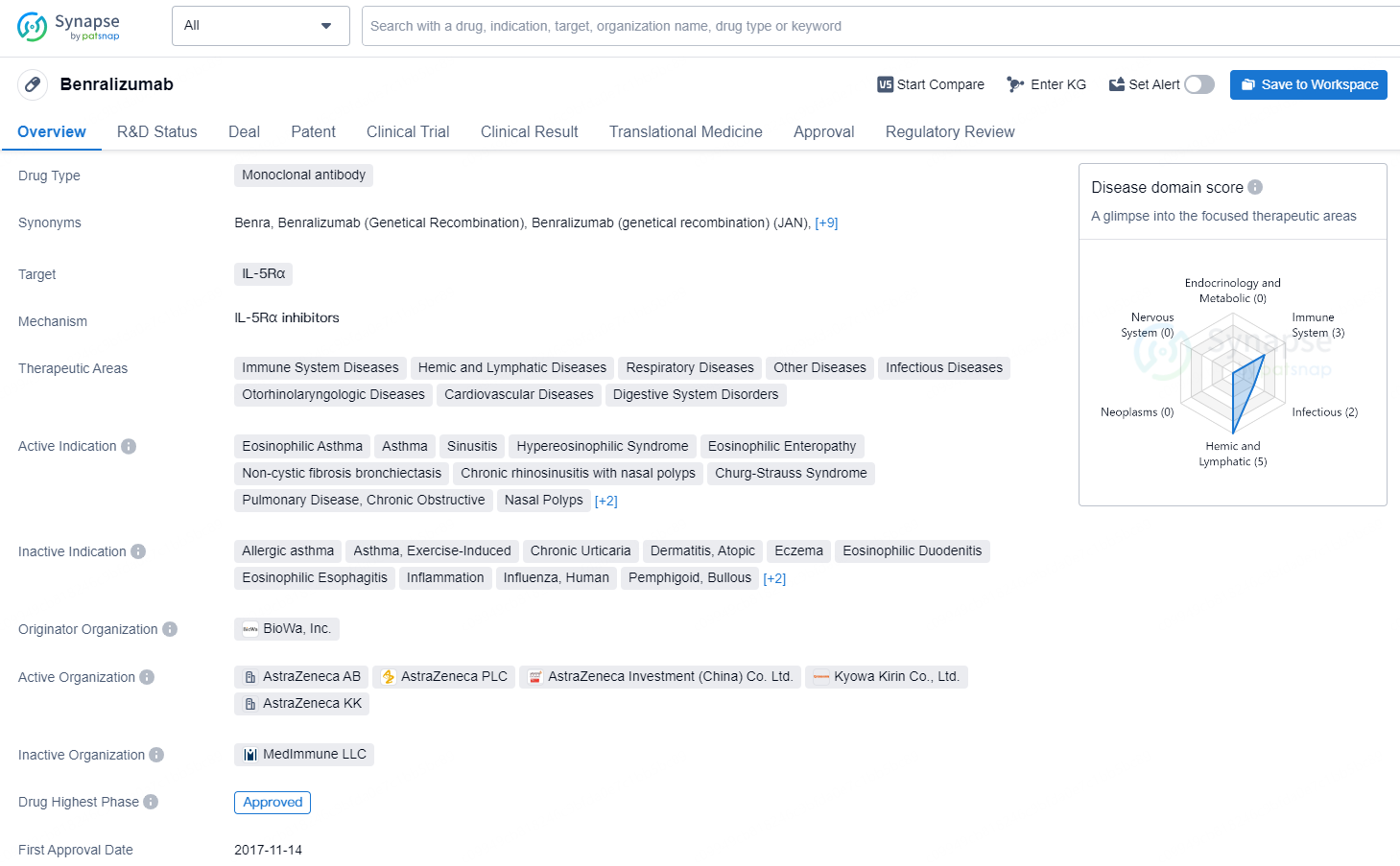
Benralizumab is a monoclonal antibody drug developed by BioWa, Inc. It targets IL-5Rα and is approved for various therapeutic areas, including immune system diseases, hemic and lymphatic diseases, respiratory diseases, infectious diseases, and others. The drug is indicated for several conditions, such as eosinophilic asthma, asthma, sinusitis, hypereosinophilic syndrome, eosinophilic enteropathy, non-cystic fibrosis bronchiectasis, chronic rhinosinusitis with nasal polyps, Churg-Strauss syndrome, pulmonary disease, chronic obstructive, nasal polyps, severe asthma, and eosinophilia.