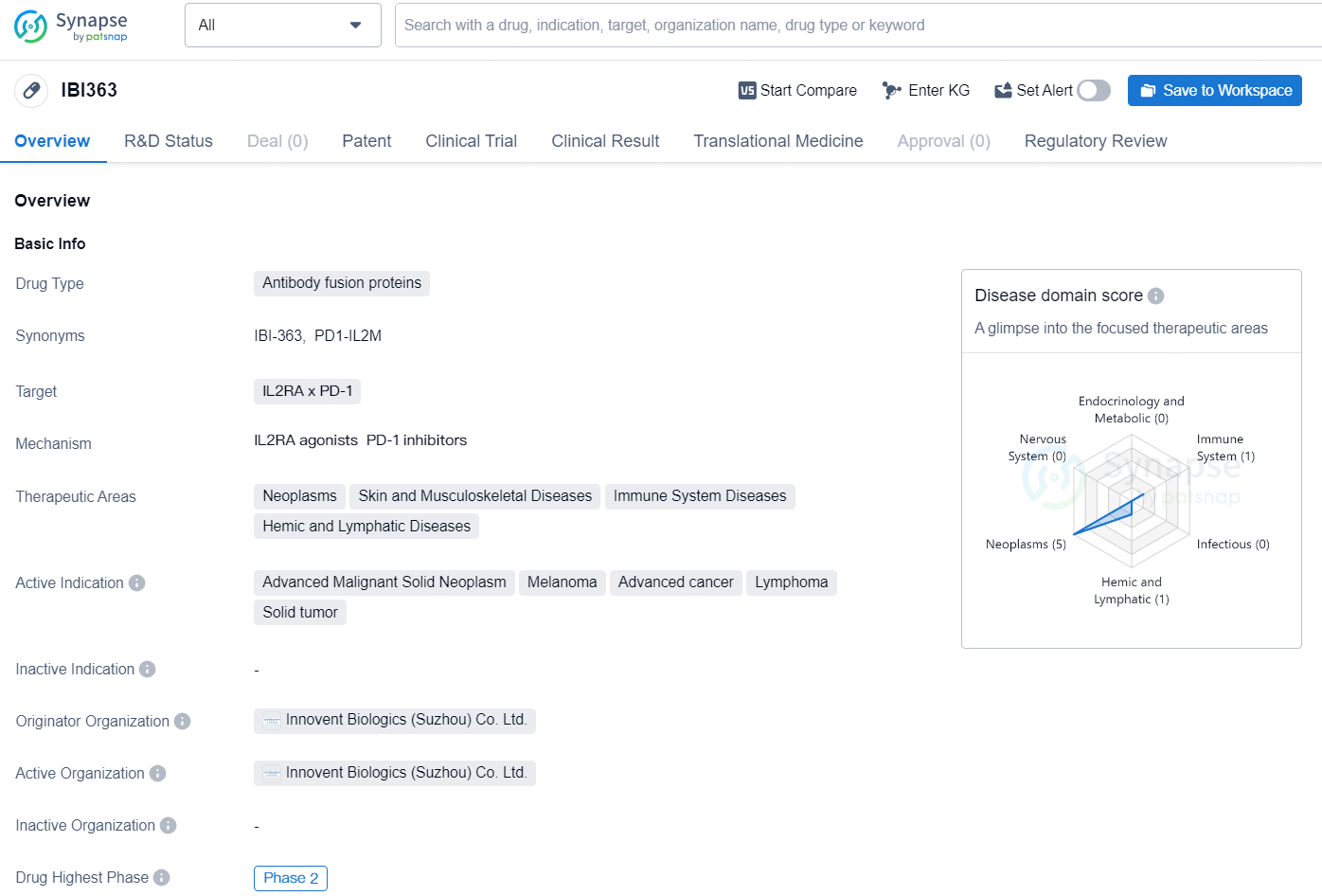
Innovative Therapy IBI363 in Advanced Colorectal Cancer: Clinical Outcomes with Bevacizumab at 2024 ESMO Congress
Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a leading global biopharmaceutical firm focused on the development, production, and commercialization of high-quality drugs for diseases such as oncology, autoimmune disorders, cardiovascular and metabolic conditions, and ophthalmology, has announced the presentation of clinical data for IBI363 (a first-in-class PD-1/IL-2α-bias bispecific antibody fusion protein) in combination with bevacizumab for advanced colorectal cancer at the 2024 ESMO Congress. Innovent is currently undertaking Phase 1/2 clinical trials in China, the United States, and Australia to assess the safety, tolerability, and efficacy of IBI363 in patients with advanced solid tumors.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Our Phase 1 trial aimed to assess the safety, tolerability, and preliminary effectiveness of the first-in-class PD-1/IL-2α-bias bispecific antibody fusion protein IBI363 in combination with bevacizumab for patients with advanced colorectal cancer (CRC).
A cohort of 35 patients was treated with the IBI363 and bevacizumab combo, showing promising anti-tumor results, with favorable safety and tolerability.
Up to the data cutoff (August 30, 2024), we treated 35 advanced CRC patients at three dose levels: 0.6 mg/kg IBI363 with 5 mg/kg bevacizumab biweekly, 1 mg/kg IBI363 with 5 mg/kg bevacizumab biweekly, and 1.5 mg/kg IBI363 with 7.5 mg/kg bevacizumab every three weeks. Of these, 91.4% were microsatellite stable (MSS) or had proficient mismatch repair (pMMR) status, with 8.6% having an unknown MSI/MMR status. A significant 91.4% had undergone two or more previous systemic therapies, 51.4% had liver metastases, 25.7% received prior immunotherapy, and 40% had mutations in KRAS/NRAS exon 2/3/4.
Common treatment-related adverse events (TRAEs) included joint pain, thyroid issues, and skin rashes. The total incidence of grade 3 or higher TRAEs was 22.9%, while grade 3 or higher immune-related adverse events (irAEs) occurred in 5.7% of patients. The safety profile of this combination treatment was in line with IBI363 monotherapy, with no new safety concerns detected.
The combination therapy showed promising anti-tumor activity in patients with MSS/pMMR CRC, with durable responses suggesting potential long-term benefits.
By the cutoff, 32 patients were considered efficacy evaluable with at least one tumor assessment post-baseline. The objective response rate (ORR) was 21.9% (confirmed ORR at 15.6%), and the disease control rate (DCR) was 65.6%. The median duration of response (DoR) was 8.1 months (95% CI: 1.5~8.2), the median progression-free survival (PFS) follow-up was 7.6 months (95% CI: 4.0~9.4), and the median PFS was 4.1 months (95% CI: 1.7~8.1). The median overall survival (OS) had not been reached.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
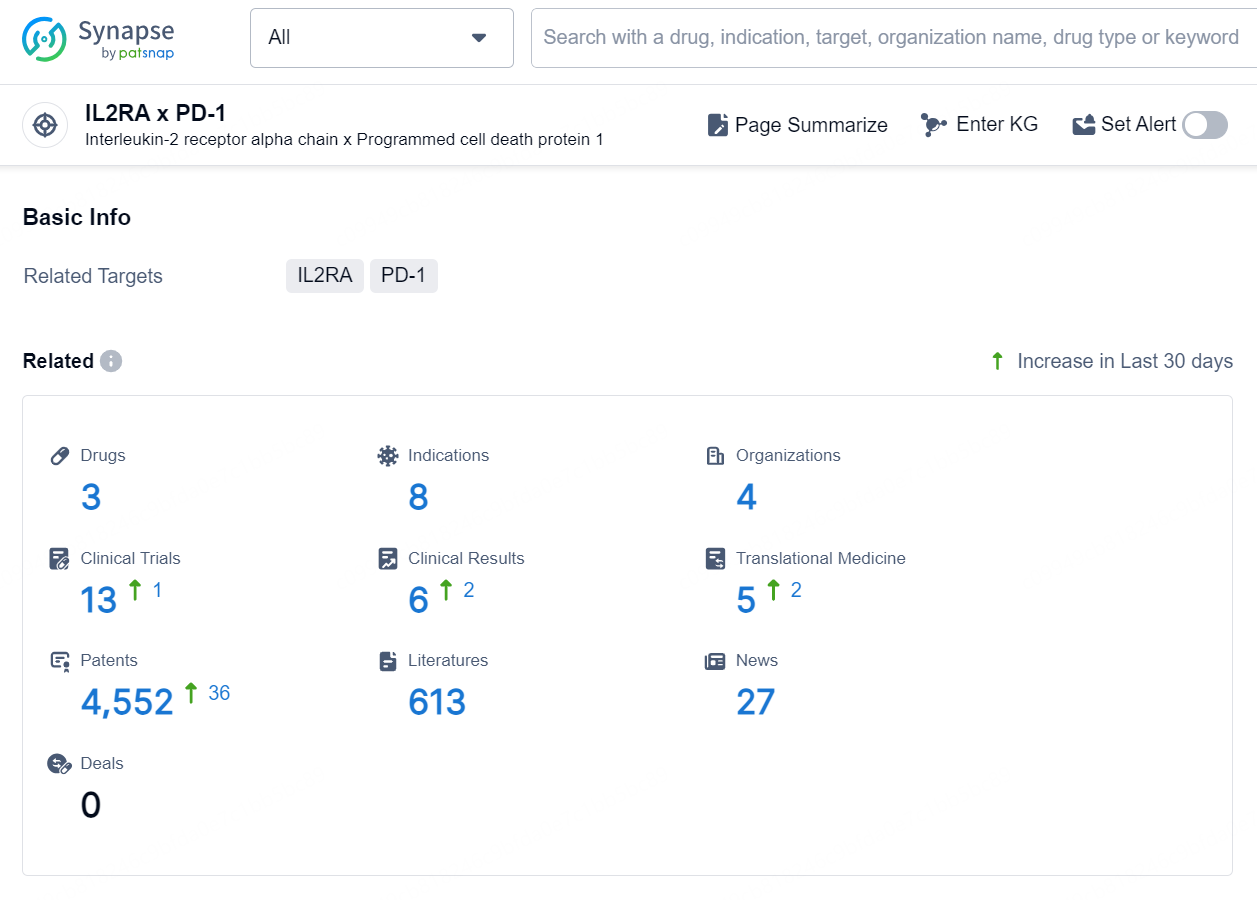
According to the data provided by the Synapse Database, As of September 18, 2024, there are 3 investigational drugs for the IL2RA and PD-1 targets, including 8 indications, 4 R&D institutions involved, with related clinical trial3 reaching 13, and as many as 4552 patents.
IBI363 is an antibody fusion protein drug developed by Innovent Biologics (Suzhou) Co. Ltd. The drug targets IL2RA and PD-1 and falls under the therapeutic areas of neoplasms, skin and musculoskeletal diseases, immune system diseases, and hemic and lymphatic diseases. The active indication for IBI363 includes advanced malignant solid neoplasm, melanoma, advanced cancer, lymphoma, and solid tumor.