FDA and EMA Acknowledge Marstacimab Filing for Hemophilia A and B Therapy
Pfizer Inc. has reported that the FDA has given the green light for the review of the firm's Biologics License Application concerning marstacimab, an anti-TFPI therapy proposed for patients diagnosed with either hemophilia A or B, who have not developed inhibitors against Factor VIII or IX. Furthermore, the application for marketing approval of marstacimab in Europe has successfully met the required standards and is presently undergoing assessment by the European Medicines Agency.
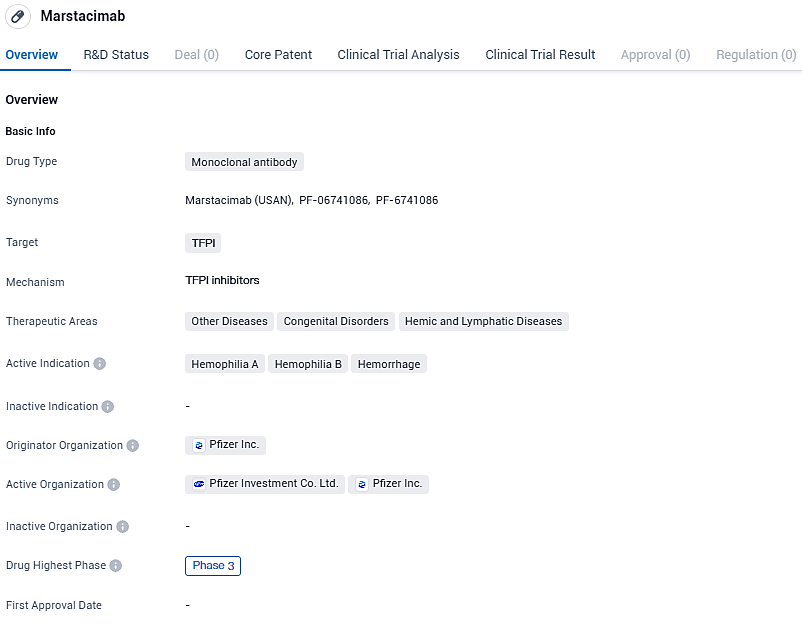
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The U.S. Food and Drug Administration (FDA) has scheduled an assessment date for the Prescription Drug User Fee Act (PDUFA) within the final quarter of 2024. Likewise, a verdict from the European Commission is forecasted for the opening quarter of 2025. Should marstacimab receive authorization in both the United States and European Union, it could emerge as the pioneering subcutaneous therapy with a once-a-week dosage for individuals affected by hemophilia B and establish a precedent for flat-dose administrations in patients with hemophilia A or B.
"Through its clinical trial outcomes, marstacimab shows promising potential as an effective treatment that employs a flat-dose, subcutaneous delivery using an auto-injector pen weekly for suitable patients," stated Dr. James Rusnak, the Chief Scientific Officer in the departments of Internal Medicine and Infectious Diseases at Pfizer's Research and Development division. "We are eager to advance the discussions of this groundbreaking treatment with the FDA, European Medicines Agency (EMA), and health regulatory bodies across the globe to deliver this significant drug to patients on an international scale."
For over 50 years, the primary therapeutic strategy for hemophilia A and B has been to administer factor replacement therapies. These therapies supplement deficient clotting factors to enable efficient blood coagulation.i The aspiration is to use a once-per-week, subcutaneous flat-dose protocol to avert severe bleeding episodes for patients diagnosed with hemophilia A and B who are deemed suitable for such treatment.
The applications for marstacimab are underpinned by the positive outcomes on both its efficacy and safety profile observed during the Phase 3 BASIS clinical trial. The critical results of this study were showcased at the prestigious American Society of Hematology Annual Meeting, which took place on December 9, 2023. The enrollment for the inhibitor group within the BASIS trial is complete, and its results are anticipated to be available by the latter part of 2024.
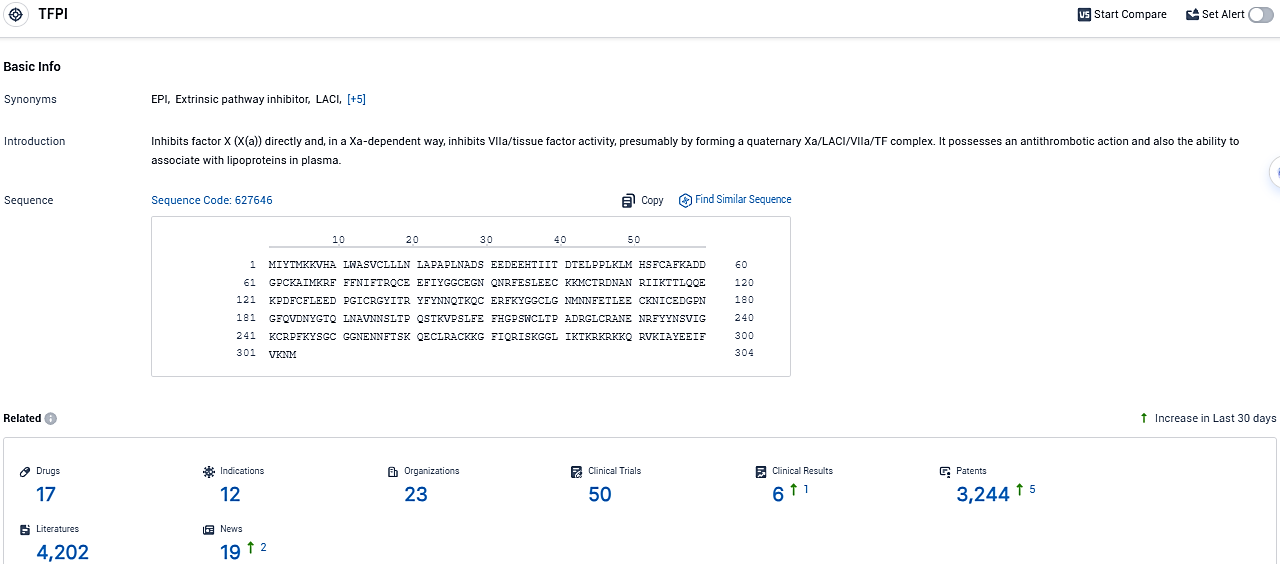
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of December 16, 2023, there are 17 investigational drugs for the TFPI target, including 12 indications, 23 R&D institutions involved, with related clinical trials reaching 50, and as many as 3244 patents.
Marstacimab is a human monoclonal immunoglobulin G isotype, subclass 1 that targets the Kunitz 2 domain of tissue factor pathway inhibitor, a natural anticoagulation protein that functions to prevent the formation of blood clots. Marstacimab is in development as a prophylactic treatment to prevent or reduce the frequency of bleeding episodes in individuals with hemophilia A or hemophilia B with or without inhibitors.