FDA Approves Bio-Thera's Phase II Trial of Novel ADC BAT8006
Bio-Thera Solutions, an enterprise in the biopharmaceutical industry currently selling products and focusing on the development of novel drug therapies and biosimilar products, has reported that it has secured permission from the US FDA to initiate a Phase II clinical trial for BAT8006. This experimental Antibody Drug Conjugate specifically targets Folate Receptor α. The upcoming trial for Phase II is set to explore the efficacy of BAT8006 in treating patients with types of cancer that are unresponsive to platinum-based chemotherapy, including ovarian cancer of epithelial origin, cancer of the fallopian tubes, and cancer starting in the peritoneum.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Folate receptor alpha (FRα), identified as a glycoprotein that binds to folic acid, is predominantly upregulated in numerous solid cancers, including those affecting the ovaries, lungs, and breasts. However, its presence in normal tissues is markedly low in both distribution and expression levels. The pronounced variance in FRα expression profiles between malignant and non-malignant tissues presents it as a compelling candidate for oncology medication development.
Utilizing proprietary technology, Bio-Thera has formulated BAT8006, an anticancer agent that marries their own anti-FRα monoclonal antibody with an innovative ADC linker-payload system. This system comprises a linker that boasts systemic stability and can be cleaved, paired with a potent small molecule inhibitor of topoisomerase I.
In early experiments, BAT8006 has shown promising results, displaying exceptional stability, non-toxicity, and robust anti-cancer properties. Its ability to carry the topoisomerase I inhibitor enables it to penetrate cellular membranes effectively, permitting the destruction of additional tumor cells around the initial targets due to a bystander killing effect. Such an effect is beneficial for managing the complex nature of tumor heterogeneity.
Presently, BAT8006 is under examination in a phase I clinical trial in China. Completion of the dose escalation phase has been achieved, and further investigations to refine the dosing parameters for various tumor types are in progress. Early results suggest that BAT8006 might lead its class of therapeutic compounds. Future conferences will shed more light on the advancement of this drug with the release of more comprehensive clinical data.
As a pioneer in pioneering antibody research and refinement, the company has successfully propelled numerous drug candidates into the final stages of development, including three drugs that have already received market approval: QLETLI® in China, plus TOFIDENCE™/BAT1806 and Avzivi®/Pobevcy® in both the United States and China. Beyond these, the company is actively testing over 20 promising entities in clinical studies, primarily focusing on immune-oncology innovations in the wake of PD-1 inhibitors and other specialized treatments like ADCs.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
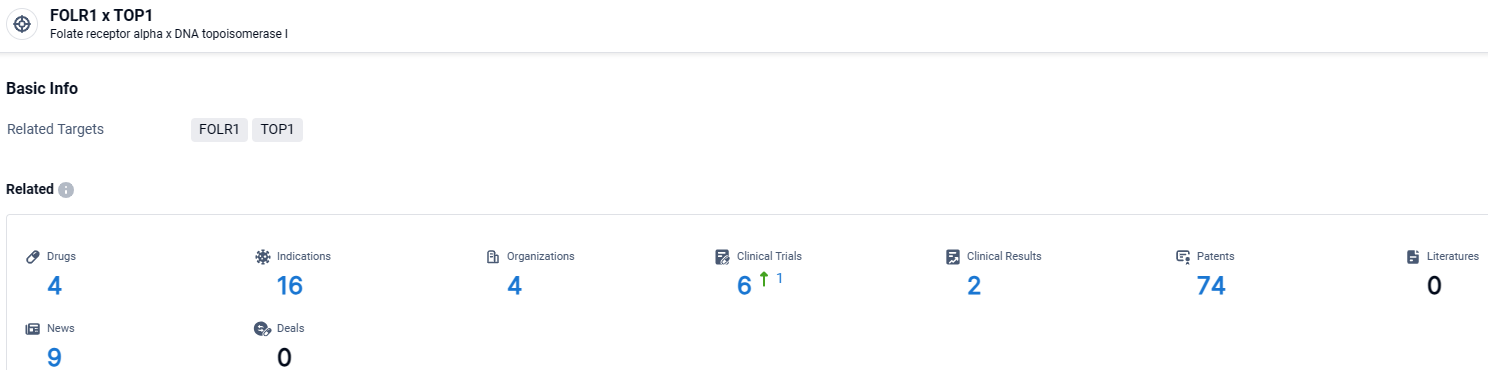
According to the data provided by the Synapse Database, As of March 26 2024, there are 4 investigational drugs for the FOLR1 and TOP1 target, including 16 indications, 4 R&D institutions involved, with related clinical trials reaching 6, and as many as 74 patents.
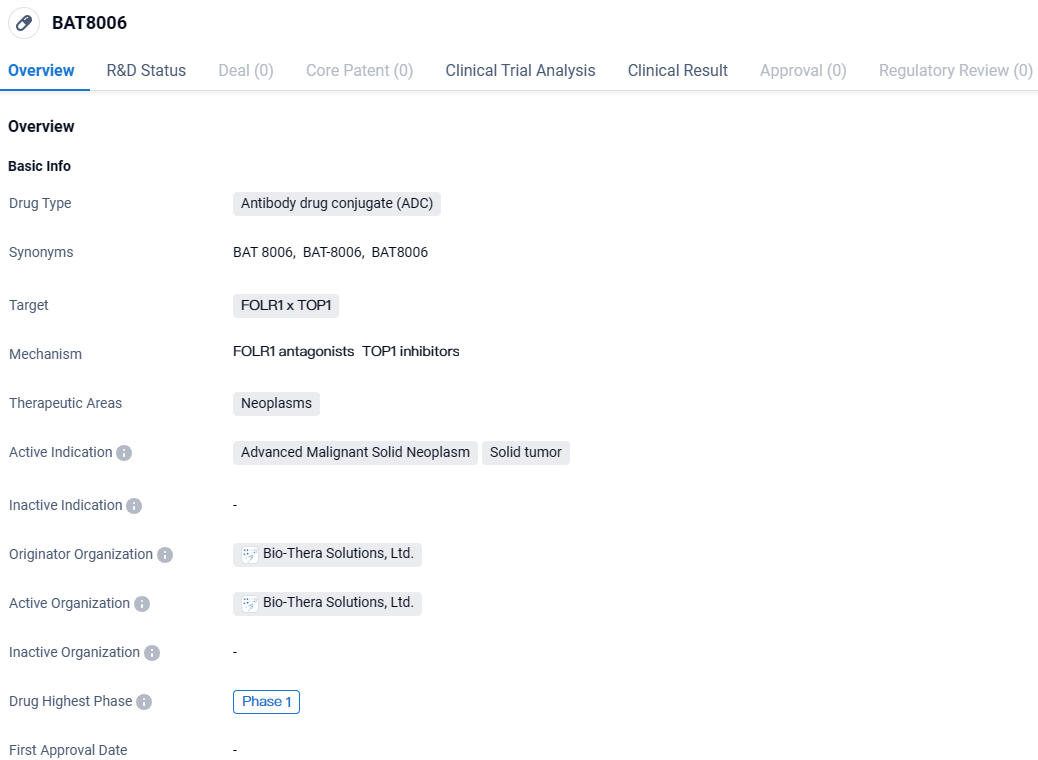
BAT8006 is an ADC being developed by Bio-Thera Solutions, Ltd. It targets FOLR1 and TOP1 and is intended for the treatment of neoplasms, specifically advanced malignant solid neoplasms and solid tumors. Currently in Phase 1 of clinical trials globally and in China, BAT8006 is still in the early stages of development, and further research is needed to determine its efficacy and safety.