Ferrer and Verge Genomics Partner to Advance ALS Experimental Treatment VRG50635 to Clinical Trials
Verge Genomics, a prominent biotech firm in the clinical phase, and Ferrer, a globally recognized B Corp entity with growing specialization in uncommon neurological conditions, recently unveiled a joint venture aimed at advancing VRG50635. As the primary therapeutic compound developed by Verge, VRG50635 targets both sporadic and inherited types of amyotrophic lateral sclerosis. The partnership focuses on the development and commercialization of this novel drug across multiple regions including Europe, Central and South America, Southeast Asia, and Japan.
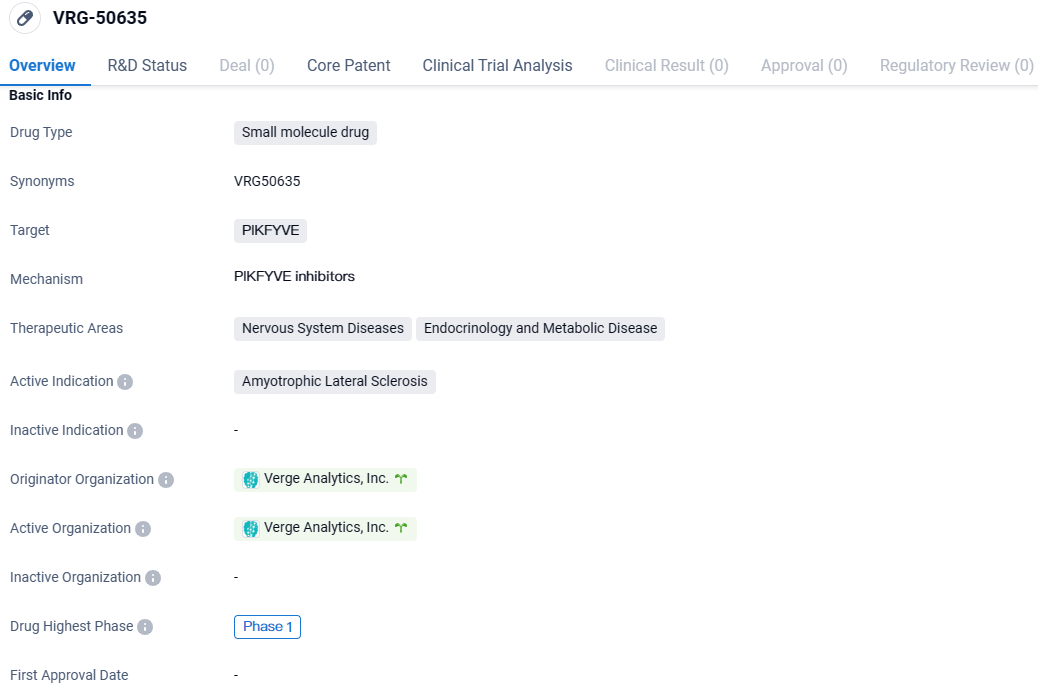
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
VRG50635 stands as an innovative and potentially superior contender within the realm of small molecule inhibitors, targeting PIKfyve, an identified point of therapeutic intervention for ALS ascertained within afflicted human specimens via Verge's CONVERGE technology—an AI-driven, humanity-centered platform.
The strategic partnership unites Verge's prowess in human-centric target identification and pharmaceutical advancement with its forward-thinking approach to clinical trials. Meanwhile, Ferrer brings its international acumen in clinical progression, production, and market introduction.
Per the partnership's framework, Ferrer secures exclusive privileges to jointly advance and market VRG50635 for the treatment of ALS in various territories beyond the USA. Verge retains comprehensive entitlements for both development and market introduction of VRG50635 across the USA and any nation not covered by this agreement.
Mario Rovirosa, CEO of Ferrer, shared his enthusiasm for the alliance with Verge Genomics, expressing the significant impact this partnership promises for individuals dealing with ALS, alongside their support networks. He voiced the union's alignment with Ferrer's dedication to deploying business as a tool in the pursuit of societal betterment, underscored by a renewed pledge to deliver transformative care options to those battling severe and incapacitating illnesses.
At Ferrer, Oscar Pérez, the Chief Scientific Officer, regards VRG50635 as a beacon of hope for a novel treatment avenue for this devastating malady. He anticipates that joining forces with Verge will hasten the progression of this potential therapy. This collaboration is deemed a crucial expansion of Ferrer's specialized medical arsenal against rare neurological conditions and serves as a reconfirmation of Ferrer's unshakeable dedication to the scientific community and individuals living with ALS.
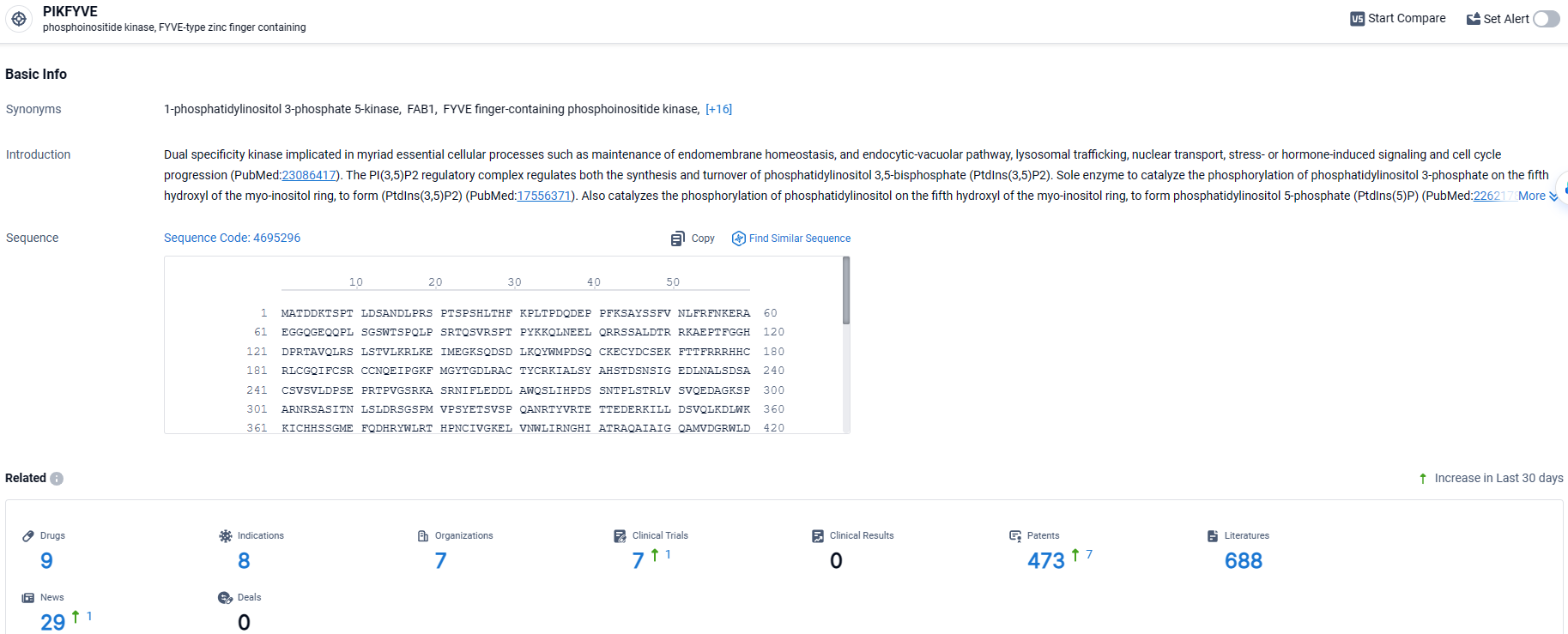
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of March 26 2024, there are 9 investigational drugs for the PIKFYVE target, including 8 indications, 7 R&D institutions involved, with related clinical trials reaching 7, and as many as 473 patents.
VRG-50635 targets PIKFYVE. It is being developed for the treatment of ALS, a neurodegenerative disease affecting the nervous system. Currently in Phase 1, further research and clinical trials are needed to evaluate its potential as a therapeutic option for ALS patients.