FDA Approves Bristol Myers Squibb's Breyanzi for Relapsed/Refractory Mantle Cell Lymphoma
Bristol Myers Squibb has revealed that the U.S. Food and Drug Administration has given the green light to Breyanzi® (lisocabtagene maraleucel; liso-cel), a CD19-directed chimeric antigen receptor T cell therapy. This therapy is meant for adult patients dealing with relapsed or refractory mantle cell lymphoma who have previously undergone a minimum of two systemic therapy regimens, one of which must have involved a Bruton tyrosine kinase inhibitor.
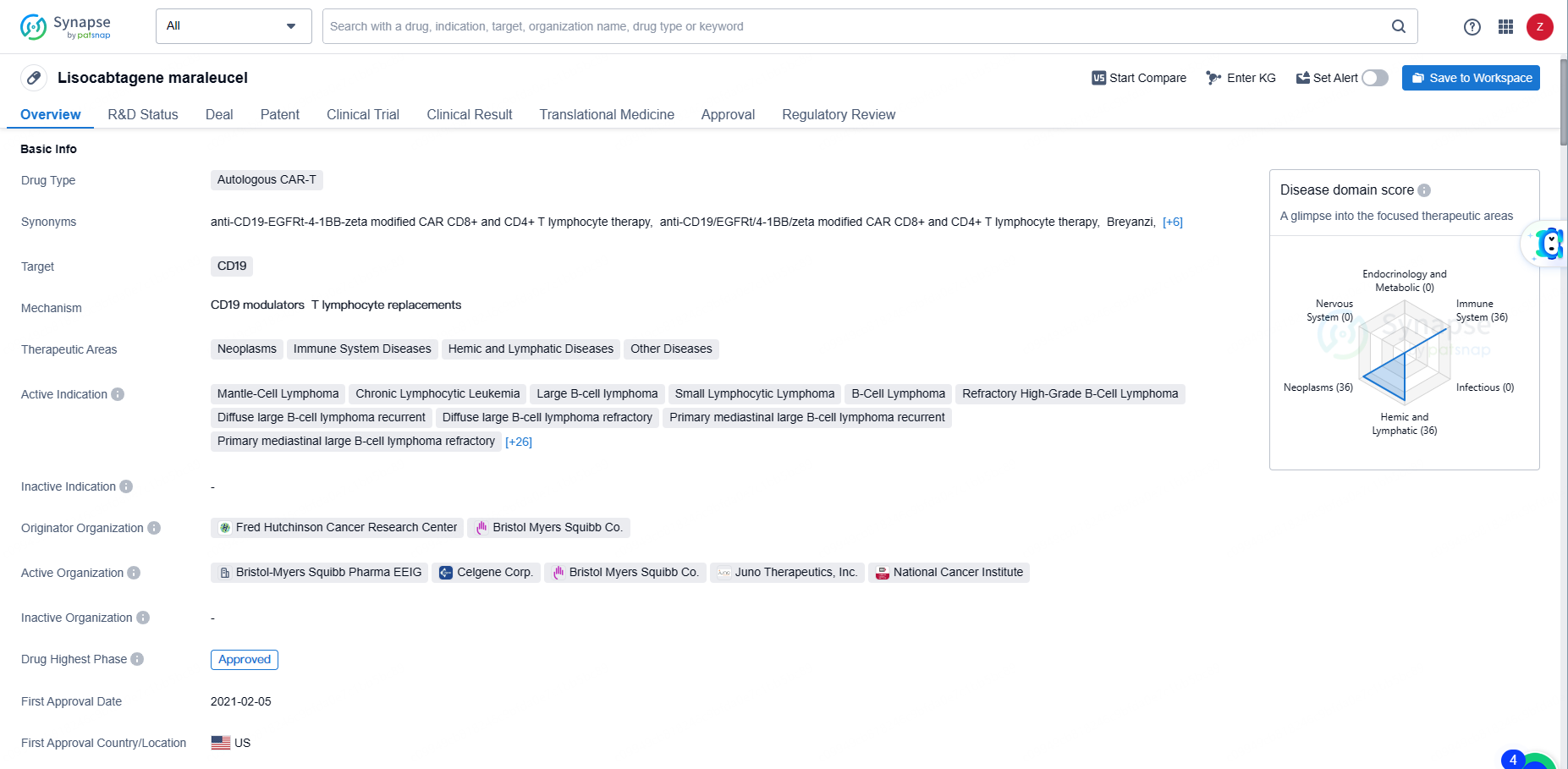
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
This FDA approval signifies that Breyanzi is now authorized for its fourth unique subtype of non-Hodgkin lymphoma, extending its applicability as a CAR T cell therapy for a wide range of B-cell malignancies. For patients with relapsed or refractory mantle cell lymphoma (MCL), Breyanzi is administered as a single infusion, encompassing a dose of 90 to 110 x 10^6 CAR-positive viable T cells. Important Safety Information, including Boxed WARNINGS on Cytokine Release Syndrome, Neurologic Toxicities, and Secondary Hematological Malignancies, can be found below.
"With Breyanzi, we are fulfilling the potential of cell therapy by providing a definitive treatment option for some of the most challenging lymphomas," stated Bryan Campbell, senior vice president and Head of Commercial, Cell Therapy at Bristol Myers Squibb. "We take pride in our advancements, which enable us to offer our unique CAR T cell therapy to a broad range of patients across various indications and stages of treatment to ensure that effective treatment options are available when they are most needed."
MCL is an infrequent but highly aggressive type of non-Hodgkin lymphoma, with many patients experiencing relapse or resistance to initial therapies. MCL is currently deemed incurable, and both response rates and response duration typically diminish with each successive relapse.
"There have been minimal advancements in treating relapsed or refractory MCL, and prognosis deteriorates for patients with every subsequent relapse, often resulting in a substantial disease burden and difficulty achieving sustained responses," commented Dr. Michael Wang, lead investigator and Puddin Clarke Endowed Professor in the Department of Lymphoma and Myeloma at the University of Texas MD Anderson Cancer Center. "The approval of Breyanzi offers a significant new CAR T treatment alternative with high rates of durable responses and a consistent safety profile, which is crucial for these patients who presently have minimal therapeutic options for this aggressive disease."
"The approval of Breyanzi introduces a novel CAR T cell therapy choice for patients combating relapsed or refractory MCL," mentioned Meghan Gutierrez, chief executive officer of the Lymphoma Research Foundation. "Each therapeutic breakthrough represents meaningful progress in enhancing patient outcomes, and this development builds on that progress by providing a potentially transformative treatment where treatment options are limited. We are grateful to the families and researchers whose efforts have made this approval a reality for those affected by this disease."
Breyanzi is comprehensively covered by commercial and governmental insurance plans in the U.S. Bristol Myers Squibb offers various programs and resources aimed at addressing the needs of patients and caregivers, facilitating access to treatments including Breyanzi. Additionally, Bristol Myers Squibb supports the patient and physician treatment experience through Cell Therapy 360, a digital platform that enhances access to pertinent information, manufacturing updates, and support for patients and caregivers.
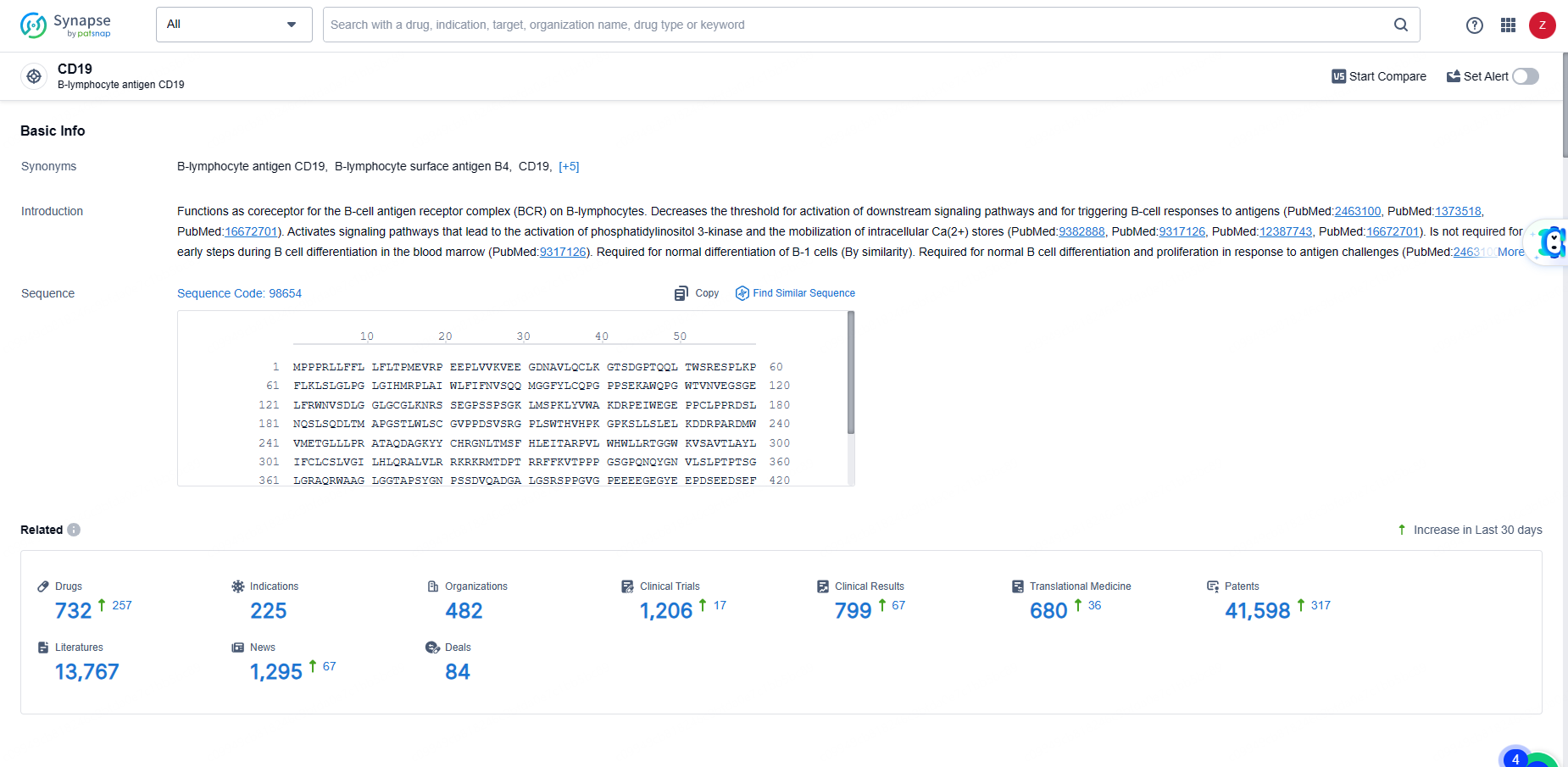
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of June 5, 2024, there are 732 investigational drugs for the CD19 target, including 225 indications, 482 R&D institutions involved, with related clinical trials reaching 1206, and as many as 41598 patents.
Lisocabtagene maraleucel represents a significant advancement in the field of biomedicine, particularly in the area of CAR-T therapy for various forms of lymphoma and leukemia. Its approval and regulatory designations underscore its potential to provide meaningful benefits to patients with these challenging conditions.