FDA Approves CARsgen's CT071 for Recurrent Multiple Myeloma and Primary Plasma Cell Leukemia Post-Therapy Failure
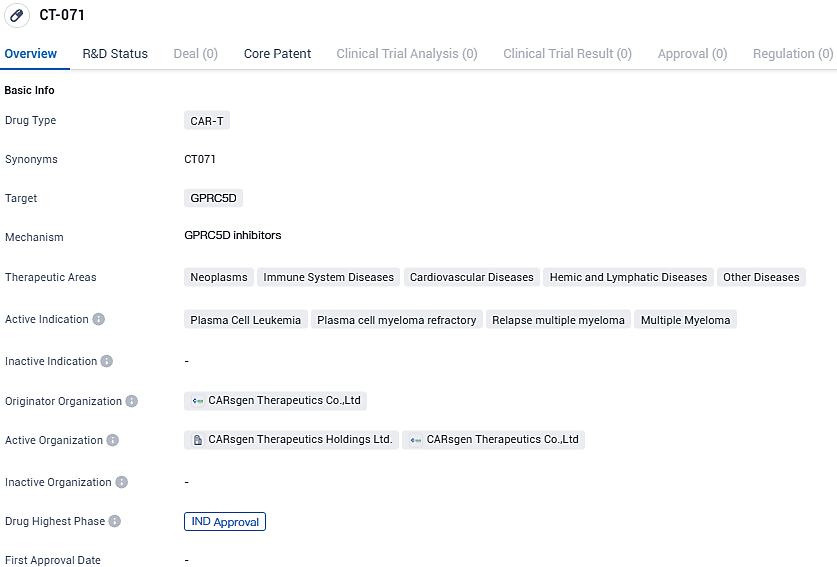
CARsgen Therapeutics Holdings Limited, a firm specializing in the development of pioneering CAR T-cell treatments targeting blood cancers and solid neoplasms, has declared the receipt of FDA's approval for its Investigational New Drug (IND) application for CT071. This investigational autologous CAR T-cell treatment is designed to home in on G protein-coupled receptor class C group 5 member D (GPRC5D). The clearance paves the way for clinical studies in individuals suffering from multiple myeloma or primary plasma cell leukemia that has either relapsed or resisted previous treatments.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
GPRC5D has come to prominence as a key focus for therapeutic intervention in the realm of multiple myeloma (MM), a prevalent yet untreatable type of blood cancer defined by rampant growth of plasma cells. The significant upregulation of GPRC5D on the surface of cancerous plasma cells, along with its sparse distribution in normal bodily tissues, positions GPRC5D as a prime molecule for therapeutic strategies aimed at combating MM and plasma cell leukemia (PCL).
Developed by CARsgen, CT071 is a therapeutic construct bearing a fully-human single-chain variable fragment (scFv) that homes in on GPRC5D with specificity. CT071 is produced utilizing CARsgen's proprietary CARcelerate™ technology. This innovative platform expedites the production process to under 48 hours, resulting in the generation of CAR T cells that are potentially more vigorous and potent due to their enhanced youth and vitality, in contrast to those created by traditional production techniques.
In China, a study initiated by a researcher has commenced to evaluate the potential risks and therapeutic benefits of CT071 in individuals with MM or PCL who have not responded to previous treatments. The initial clinical data gleaned from this Investigator-Initiated Trial (IIT) indicates a promising safety profile and suggests initial signs of therapeutic activity.
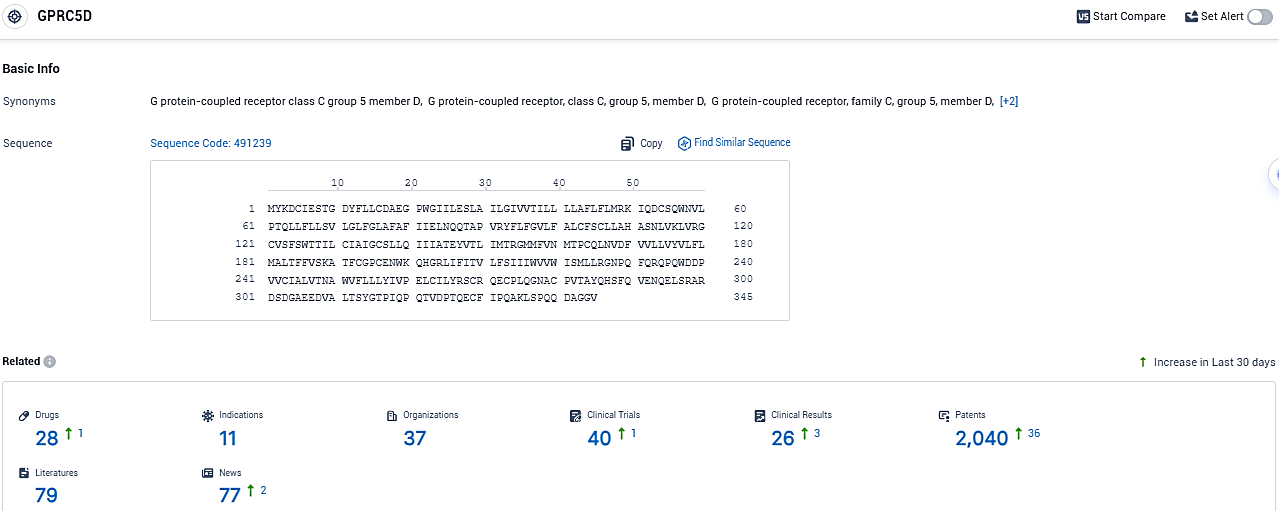
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of December 12, 2023, there are 28 investigational drugs for the GPRC5D target, including 11 indications, 37 R&D institutions involved, with related clinical trials reaching 40, and as many as 2040 patents.
CT071 is a CAR T-cell therapy candidate developed utilizing proprietary CARcelerateTM platform of CARsgen targeting GPRC5D for the treatment of relapsed/refractory MM or relapsed/refractory PCL. An IIT is ongoing in China to evaluate the safety and efficacy of CT071 for the treatment of relapsed/refractory multiple myeloma or plasma cell leukemia.