Brenig Therapeutics Reports BT-0267 LRRK2 Blocker Shows Improved Safety and Significant CSF Blood Levels at Parkinson's Symposium
Brenig Therapeutics, backed by investment firms such as Torrey Pines Investments, OrbiMed Advisors, and BioGeneration Ventures, has revealed recent findings concerning its investigational LRRK2 antagonist, BT-0267. This announcement was made during the esteemed conference dedicated to the advancements in Parkinson's Disease treatment.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
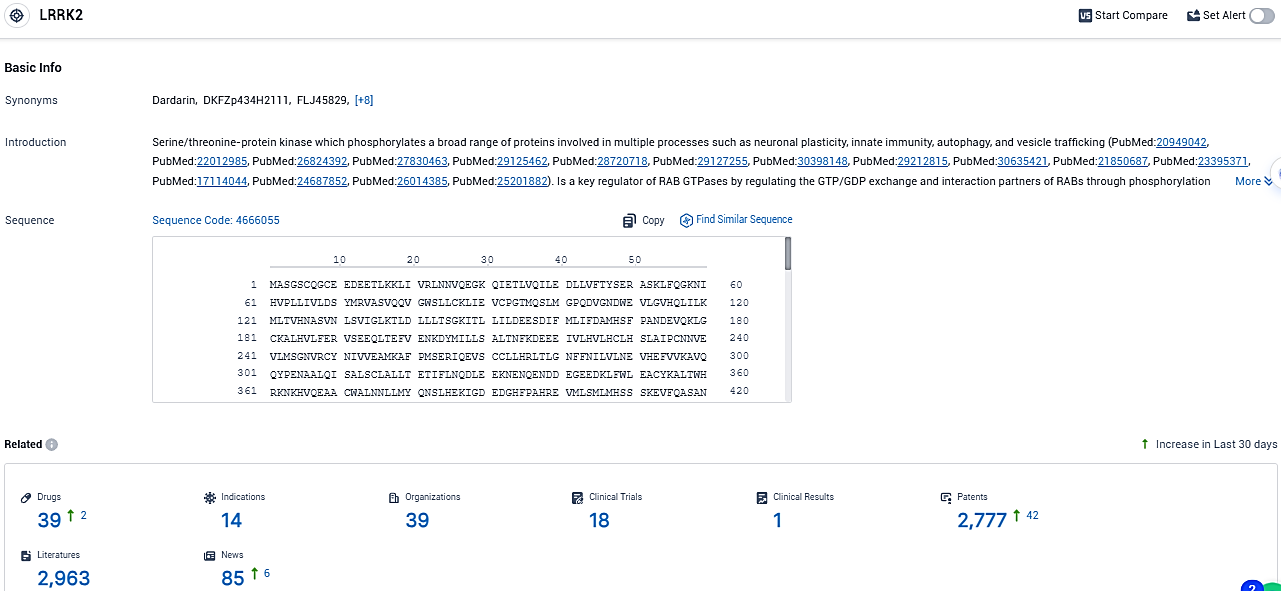
LRRK2, identified as a key therapeutic target for Parkinson’s disease, is instrumental in the disease’s development. Suppression of LRRK2 activity is increasingly viewed as an effective strategy for altering the progression of the disease, with numerous drug compounds progressing to clinical evaluation stages.
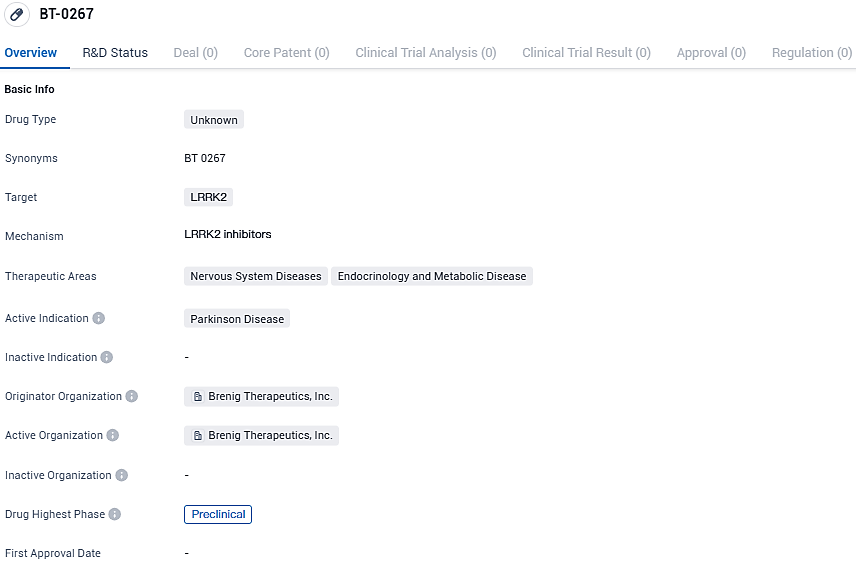
The drug candidate BT-0267 exhibits notable ability to cross the blood-brain barrier while limiting exposure to peripheral tissues, also demonstrating an enhanced safety profile overall. Experimental results showcased BT-0267’s effectiveness within the brain of live models, without detectable changes to lung and kidney structure when compared to alternative LRRK2 inhibitor options.
Moreover, BT-0267 stands out with an impressive cerebrospinal fluid (CSF) to unbound plasma ratio in studies on non-human primates, shows exceptional selectivity at 1000-fold against competing kinases, and displays greater than 500-fold selectivity over other compounds from the safety panel, evidencing its leading status in terms of safety.
Dr. Alexei Pushechnikov, the leader in chemical science and Chief Technical Officer at Brenig Therapeutics, stated, “The exceptional CSF to plasma ratio achieved with BT-0267 aligns with our systematic drug design approach that aims to mitigate adverse effects on the lungs and kidneys in the context of our LRRK2 inhibition endeavor.”
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of December 12, 2023, there are 39 investigational drugs for the LRRK2 target, including 14 indications, 39 R&D institutions involved, with related clinical trials reaching 18, and as many as 2777 patents.
BT-0267 is a drug developed by Brenig Therapeutics, Inc. in the field of biomedicine. It targets LRRK2 and has potential applications in treating nervous system diseases and endocrinology and metabolic diseases. The active indication for BT-0267 is Parkinson's disease. However, it is important to note that BT-0267 is currently in the preclinical phase of development, and further research and testing are required before its efficacy and safety can be determined.