Simcere Zaiming Announces NMPA Approval of Cetuximab Beta in China
Simcere Zaiming, a cutting-edge company focused on oncology and part of Simcere Pharmaceutical Group, announced that their new anti-epidermal growth factor receptor (EGFR) antibody treatment, Enlituo®, has been officially approved for sale by the China National Medical Administration. This advanced therapy, created in collaboration with Mabpharm Limited, is intended for use in combination with the FOLFIRI regimen as an initial treatment for RAS/BRAF wild-type metastatic colorectal cancer.
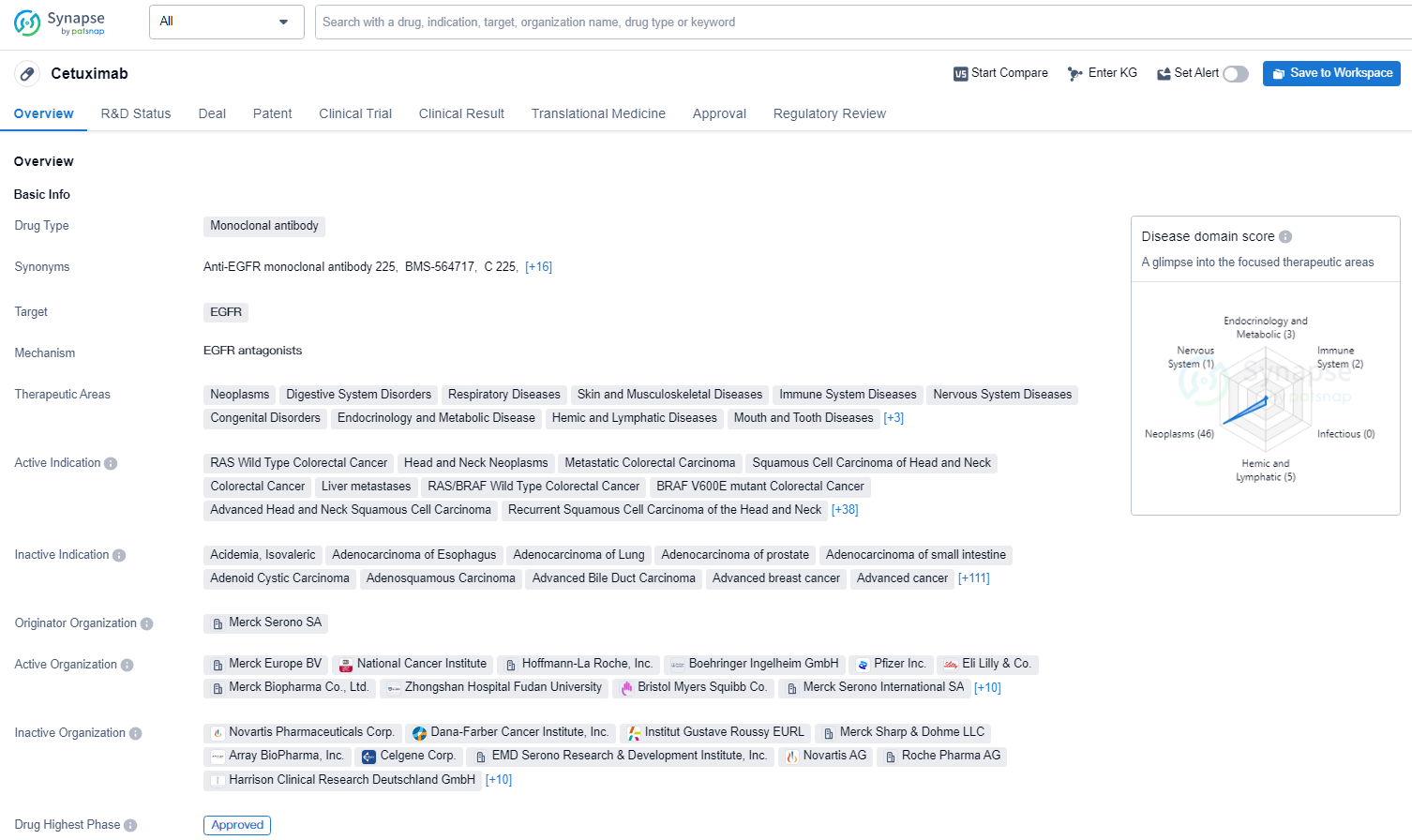
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Referring to the National Cancer Center’s 2022 report 'Cancer Incidence and Mortality in China,' colorectal cancer ranks as the second most common cancer in the country. Annually, there are 1.571 million new cases and 240,000 deaths, significantly impacting public health. When colorectal cancer metastasizes, primary treatment options include chemotherapy and targeted therapies. Prior clinical studies have demonstrated the effectiveness of EGFR-targeted antibody treatments for malignant tumors, particularly mCRC, in patients without RAS/BRAF mutations.
Enlituo® (cetuximab beta, formerly CMAB009) is a recombinant EGFR monoclonal antibody independently developed in China. Classified as a category 2.4 modified biological new drug, cetuximab beta is produced using a proprietary protein expression technique, avoiding glycosylation changes that can lead to hypersensitivity reactions.
The approval of Enlituo® was based on robust evidence from phase 2/3 and phase 3 confirmatory clinical trials. In a prospective, open-label, randomized, controlled, multicenter phase 3 trial, 505 patients with RAS/BRAF wild-type mCRC were treated and analyzed. Clinical results showed that cetuximab beta, when combined with FOLFIRI, significantly increased progression-free survival compared to FOLFIRI alone.
Dr. Renhong Tang, Chairman of Simcere Zaiming, emphasized the unique clinical benefits of Enlituo® in targeted therapy: “The primary indication for colorectal cancer covers a large patient population. This product complements Simcere Zaiming’s existing portfolio, and efforts are underway to accelerate its commercialization to benefit more cancer patients.”
Dr. Hao Wang, CEO of Mabpharm, expressed his excitement about Enlituo® addressing a gap in colorectal cancer treatment with a domestically developed EGFR-targeted therapeutic. “This provides updated treatment options for both doctors and patients. Working closely with Simcere Zaiming’s Marketing Team, we are quickly realizing the clinical promise of Enlituo®, delivering a high-quality and cost-effective biological new drug to hundreds of thousands of patients in China.”
Enlituo® is the first EGFR monoclonal antibody drug developed in China with its own intellectual property rights approved for first-line treatment of mCRC. Its successful introduction will provide Chinese cancer patients with a high-quality and affordable biological targeted therapy.
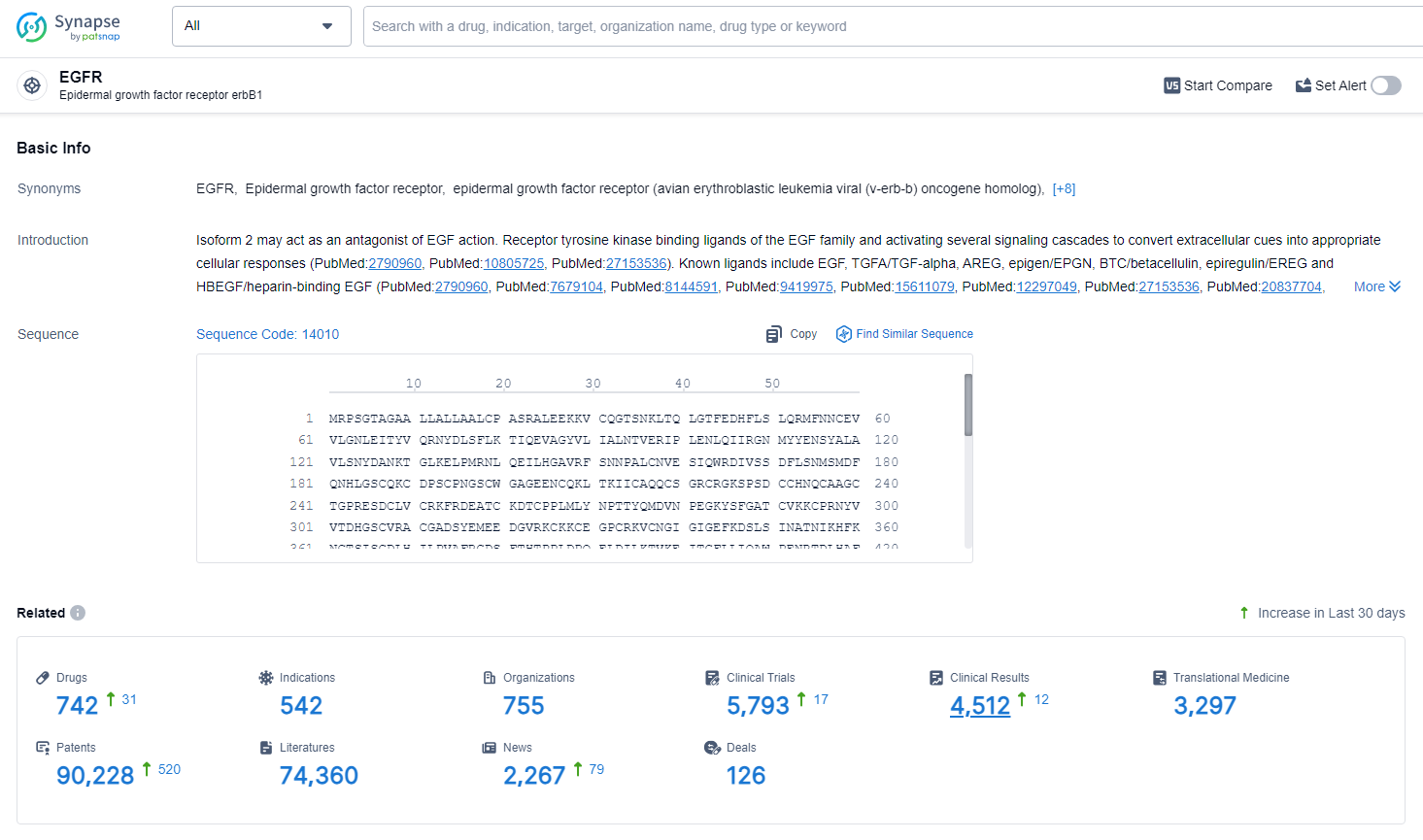
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of July 2, 2024, there are 742 investigational drugs for the EGFR target, including 542 indications, 755 R&D institutions involved, with related clinical trials reaching 5793, and as many as 90228 patents.
Cetuximab is a monoclonal antibody drug that targets the epidermal growth factor receptor (EGFR). It is used in the treatment of a wide range of therapeutic areas including neoplasms, digestive system disorders, respiratory diseases, skin and musculoskeletal diseases and other diseases. Cetuximab has demonstrated its efficacy in the treatment of a wide range of therapeutic areas and has been approved for use in multiple countries, making it an important drug in the field of biomedicine.