FDA Approves First and Only Once-Daily Single-Pill Combination Therapy for Pulmonary Arterial Hypertension
On March 22, Johnson & Johnson announced that the U.S. FDA had approved its new medication, OPSYNVI®, for the treatment of Group 1 pulmonary arterial hypertension (PAH) as classified by the World Health Organization, and for patients with WHO functional class II-III PAH in adults. OPSYNVI® is a single tablet combination therapy that contains an endothelin receptor antagonist (macitentan) and a phosphodiesterase 5 (PDE5) inhibitor (tadalafil).

PAH is a rare, progressive, and life-threatening vascular disease characterized by the narrowing of small arteries in the lungs and elevation of blood pressure in the pulmonary circulation, potentially leading to right heart failure. In the United States, there are approximately 500 to 1000 new cases of PAH each year, classifying it as a rare disease.
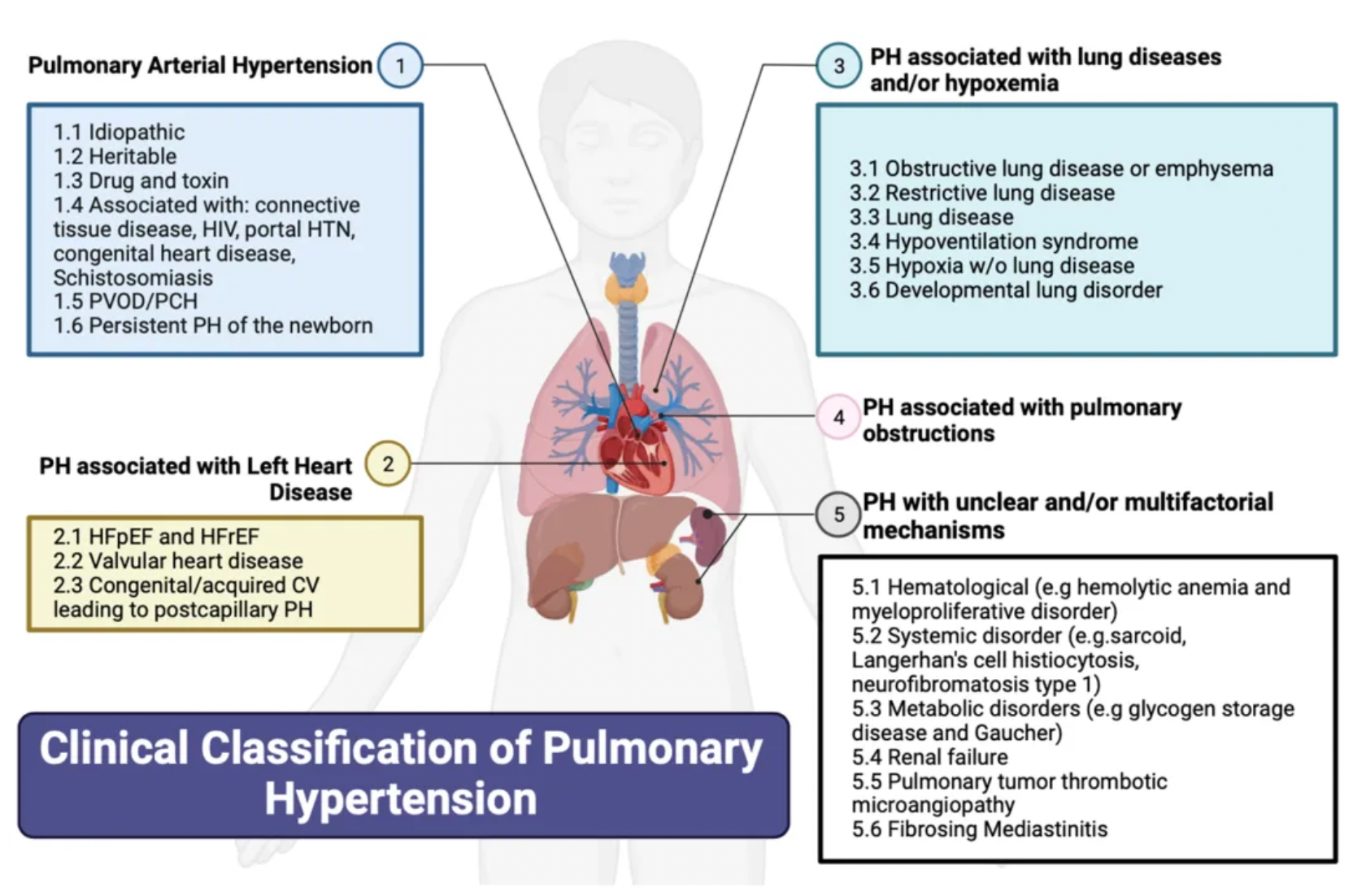
According to the classification system updated by the cause of the disease at the 6th World Symposium on Pulmonary Hypertension:
·Group 1 pulmonary hypertension (PH), also known as pulmonary arterial hypertension (PAH), encompasses various causes, including: connective tissue diseases (most commonly systemic sclerosis), HIV infection, portopulmonary hypertension due to liver diseases, drug and toxin-induced PAH, congenital heart diseases leading to systemic-to-pulmonary shunts, as well as idiopathic and heritable pulmonary arterial hypertension.
·Group 2 pulmonary hypertension (PH) is caused by left heart diseases and is also known as post-capillary or pulmonary venous hypertension. It covers issues such as left ventricular systolic dysfunction and/or diastolic heart failure, as well as heart failure associated with diseases of the valves on the left side of the heart.
·Group 3 pulmonary hypertension (PH) is induced by chronic hypoxemia and respiratory system diseases.
·Group 4 pulmonary hypertension (PH) is associated with chronic thromboembolic obstruction in the pulmonary arteries, with chronic thromboembolic pulmonary hypertension (CTEPH) being the most common manifestation.
·Group 5 pulmonary hypertension (PH) includes miscellaneous causes such as sarcoidosis, thyroid disorders, end-stage renal disease (whether treated with dialysis or not), and other diseases leading to pulmonary hypertension.
In 2022, the European Society of Cardiology (ESC) and the European Respiratory Society (ERS) published clinical guidelines recommending initial combination therapy with endothelin receptor antagonists and PDE5 inhibitors for patients with low to intermediate risk idiopathic PAH, heritable drug-related PAH, or PAH associated with connective tissue disease without cardiovascular lung complications.
The emergence of OPSYNVI®, a single-pill combination medication, not only simplifies the medication administration process for patients but also aligns more closely with the multidisciplinary treatment philosophy of current PAH guidelines, potentially improving treatment adherence and efficacy.
About Macitentan
Macitentan is a dual endothelin receptor antagonist specifically designed for the treatment of pulmonary arterial hypertension (PAH). This medication was first approved by the U.S. Food and Drug Administration (FDA) in 2013 for the purpose of alleviating disease progression in PAH patients.
Compared to its predecessor, bosentan, an important advantage of Macitentan lies in its reduced risk of hepatotoxicity, which means it has less impact on patients' livers during long-term treatment. In terms of its mechanism of action, Macitentan works by blocking endothelin receptors ETA and ETB, reducing pathological processes such as pulmonary arterial constriction, smooth muscle cell proliferation, and fibrosis. As a result, it helps to decrease pulmonary vascular resistance, improve cardiac function, prevent or delay the worsening of PAH patients' condition, and enhance their quality of life. Macitentan's role in the treatment of pulmonary arterial hypertension involves counteracting the adverse effects of the abnormal activation of the endothelin system, aiming to help control disease progression and reduce the risk of hospitalization and other clinical deteriorations due to PAH.
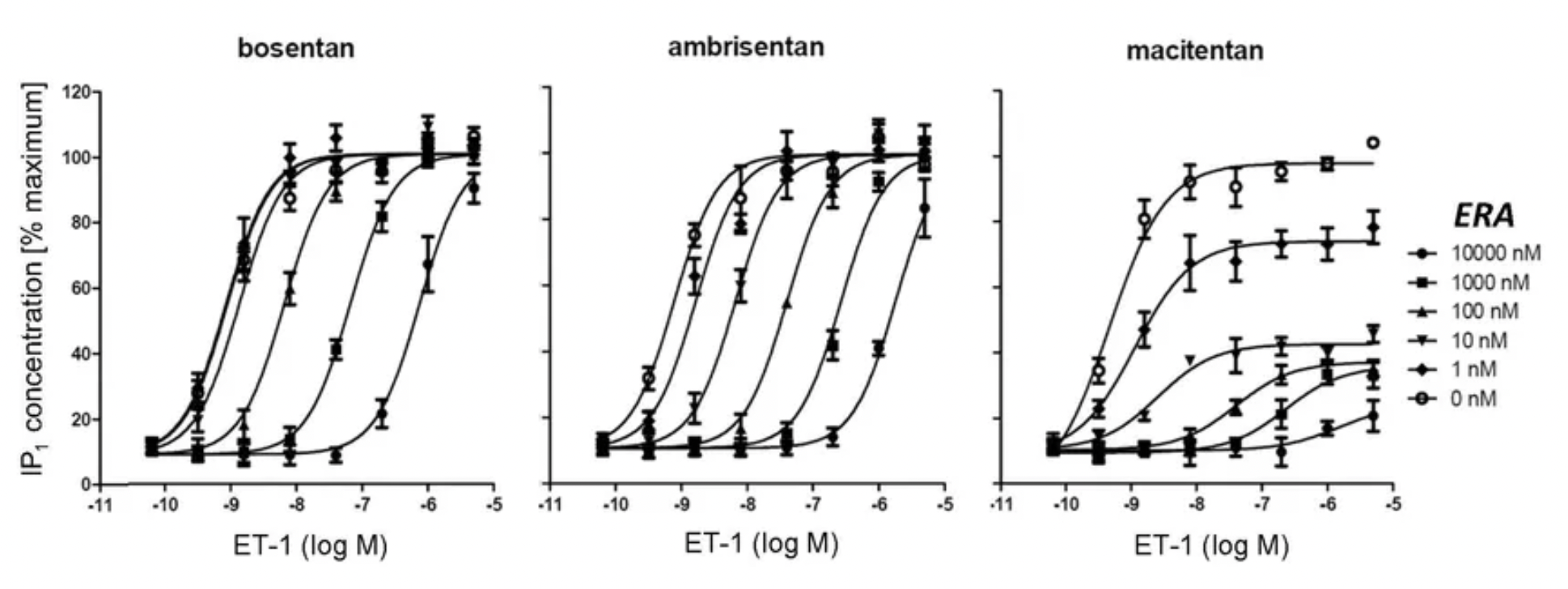
According to clinical research data published in 2024 (NCT03904693), this multicenter, double-blind, adaptive phase III A DUE study compared the efficacy and safety of a fixed-dose combination of Macitentan/Tadalafil (M/T) to monotherapy with Macitentan 10 mg and monotherapy with Tadalafil 40 mg in the treatment of pulmonary arterial hypertension patients, including those who were untreated and those who had previously only received treatment with endothelin receptor antagonists or phosphodiesterase type 5 inhibitors as monotherapy.

Research findings indicate that a total of 187 patients were randomized to either the single-pill M/T FDC group (108 patients), the Macitentan group (35 patients), or the Tadalafil group (44 patients). In comparison to Macitentan monotherapy, treatment with the M/T FDC significantly reduced pulmonary vascular resistance (PVR) by 29%, with a geometric mean ratio of 0.71; the reduction in PVR was also significant compared to Tadalafil monotherapy, amounting to a 28% decrease, with a geometric mean ratio of 0.72. There were three patient deaths in the M/T FDC treatment group, but these were judged to be unrelated to the treatment. The M/T FDC group experienced more adverse events (AEs) leading to discontinuation of the drug, serious adverse events, and adverse events of special interest (anemia, hypotension, and edema).
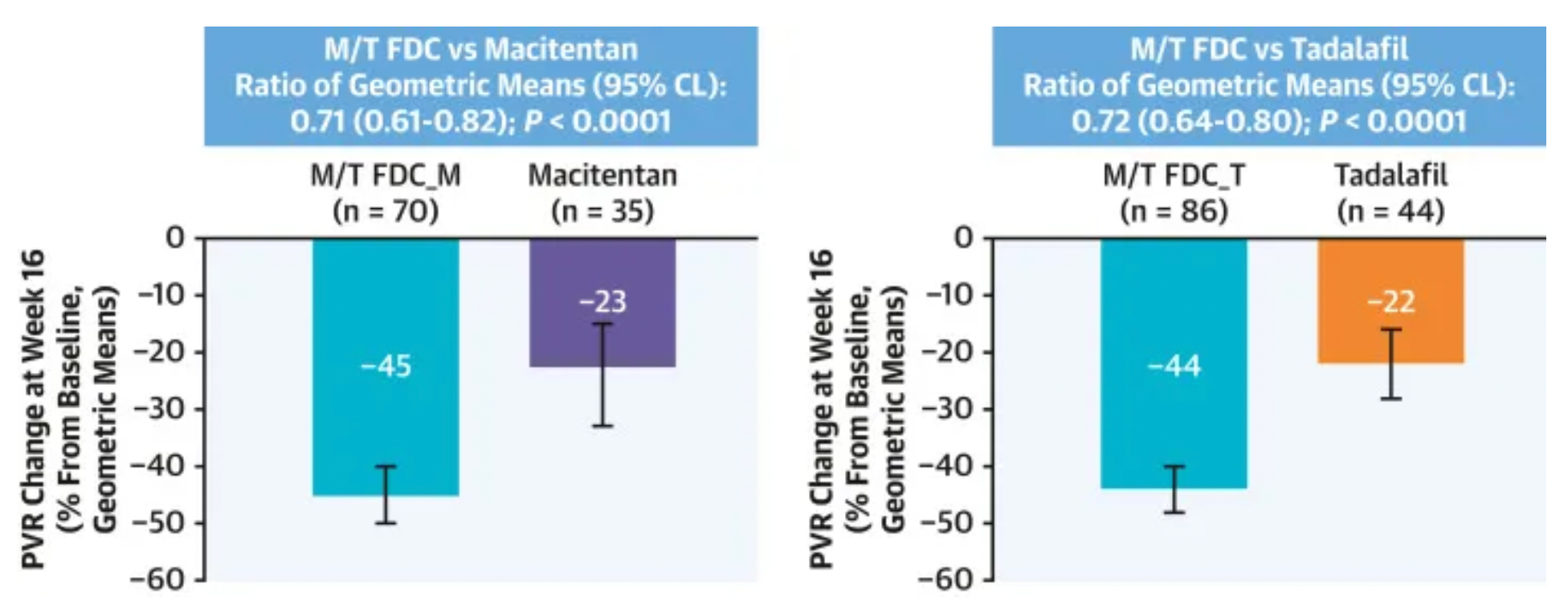
In conclusion, the fixed-dose combination therapy substantially improved the efficacy in lowering pulmonary vascular resistance in PAH patients compared to monotherapy, with safety and tolerability profiles consistent with its individual components.
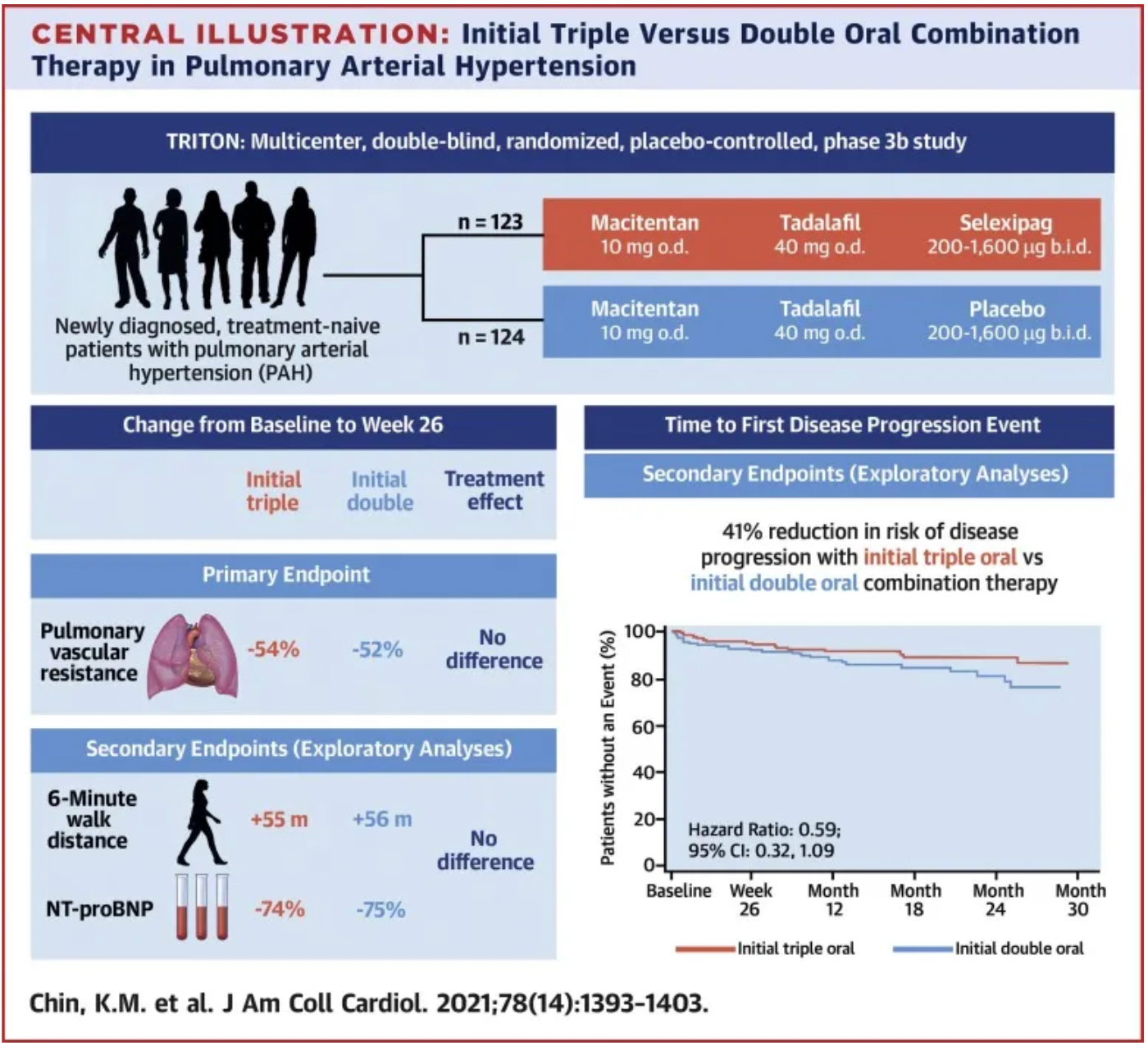
Another multicenter, double-blind, randomized phase 3b clinical study, TRITON, published in 2021, also evaluated the efficacy and safety of initial triple oral therapy (macitentan, tadalafil, and selexipag) versus initial double oral therapy (macitentan, tadalafil plus placebo) in patients newly diagnosed with pulmonary arterial hypertension (PAH) who had not previously received treatment.

The results indicated that at week 26, both treatment approaches significantly reduced the pulmonary vascular resistance in patients compared to baseline (by 54% and 52%, respectively), yet the difference in reduction between the two groups was not statistically significant. At week 26, both the initial triple-combination therapy group and the initial dual-combination therapy group showed improvements in the 6-minute walk distance and N-terminal pro-brain natriuretic peptide levels, with no notable differences between the two groups. It is noteworthy that at the end of the primary observation period, there were 2 deaths in the initial triple-combination therapy group compared to 9 deaths in the initial dual-combination therapy group. Exploratory analysis suggests that initial triple-oral therapy may offer a better long-term treatment outcome compared to initial dual therapy.
Current Status of Endothelin Receptor Antagonist Development
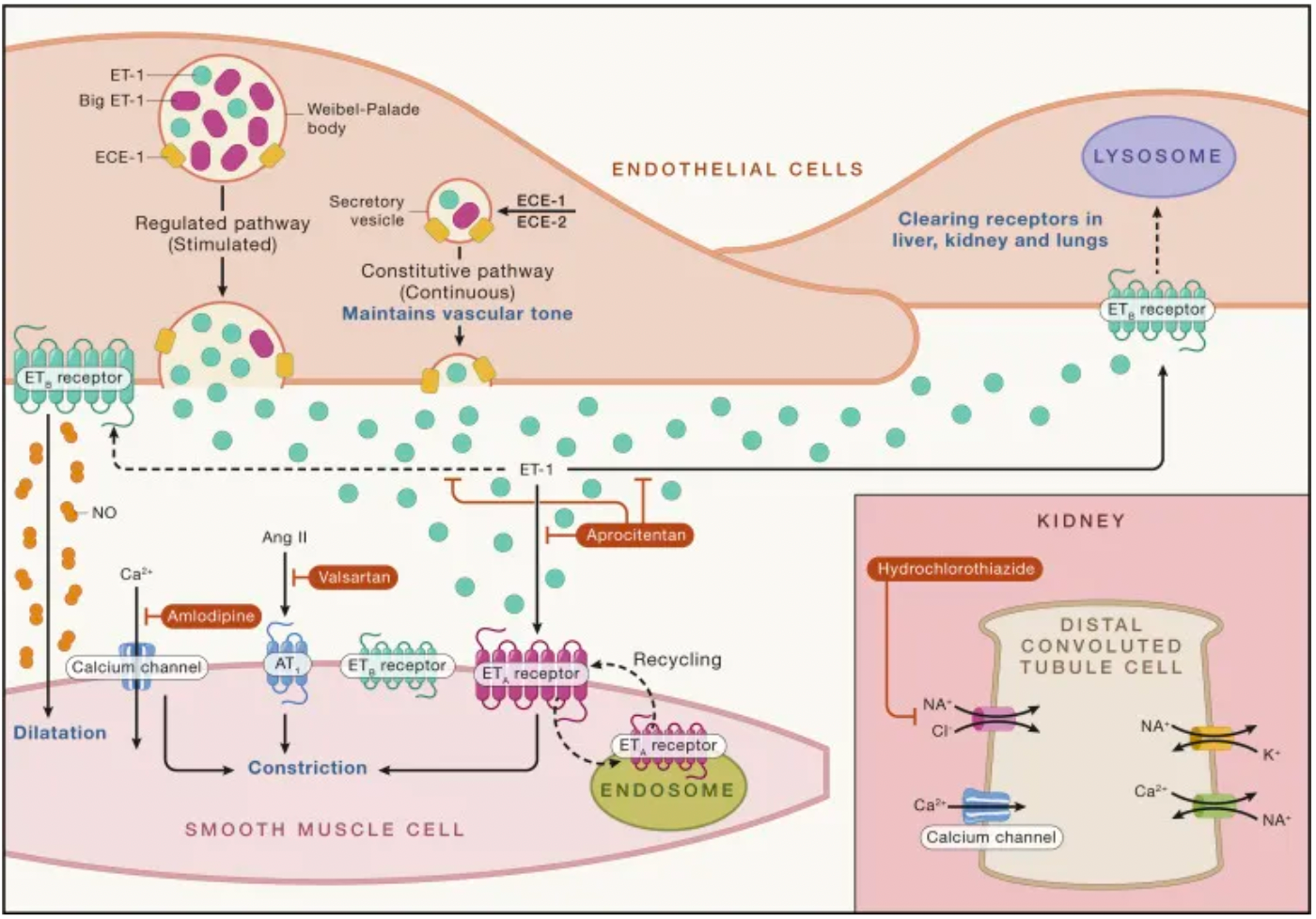
Endothelin-1 (ET-1) was identified in 1988 when Japanese scientist Yanagisawa and colleagues reported the discovery of endothelin-1, an elusive "endothelium-derived vasoconstrictor factor," which ignited the field of biomedical research.
Endothelin-1 exhibits significant pharmacological properties: it has a sustained vasoconstrictive effect on the smooth muscle of the coronary arteries, with a potency an order of magnitude greater than that of angiotensin II or other vasoactive agents. Endothelin-1 is expressed in the endothelial cells of all inspected human vascular beds. In a pioneering human study, a key finding with the use of a selective ETA receptor antagonist was that endothelin-1 maintains normal vascular tone through the ETA receptors expressed on smooth muscle cells. Conversely, the ETB receptors expressed by endothelial cells release the vasodilator nitric oxide in an autocrine manner, thereby relaxing the underlying smooth muscle.
DOI: 10.1016/j.cell.2022.12.014
ETA and ETB receptors, as key components of the endothelin system, hold significant value in clinical research and therapy. These two G-protein-coupled receptors are activated by the endothelin peptides 1-3, which are produced by endothelial cells and various other cell types, including renal cells, neurons, and macrophages. They are crucial in regulating physiological functions of the cardiovascular system, kidneys, and metabolism.
To date, the FDA has approved a total of 5 ETA or ETB antagonists, including Bosentan (2001, dual antagonist), Sitaxentan (2006, withdrawn), Ambrisentan (2007, ETA receptor-selective), Macitentan (2013, dual antagonist), and Sparsentan (2023, dual ETA+AT1R antagonist).
Bosentan (Ro-47-0203) is a potent dual antagonist for the ETA and ETB receptors, developed by the research team at Roche in Switzerland. Its activity has been studied in various preclinical disease models.
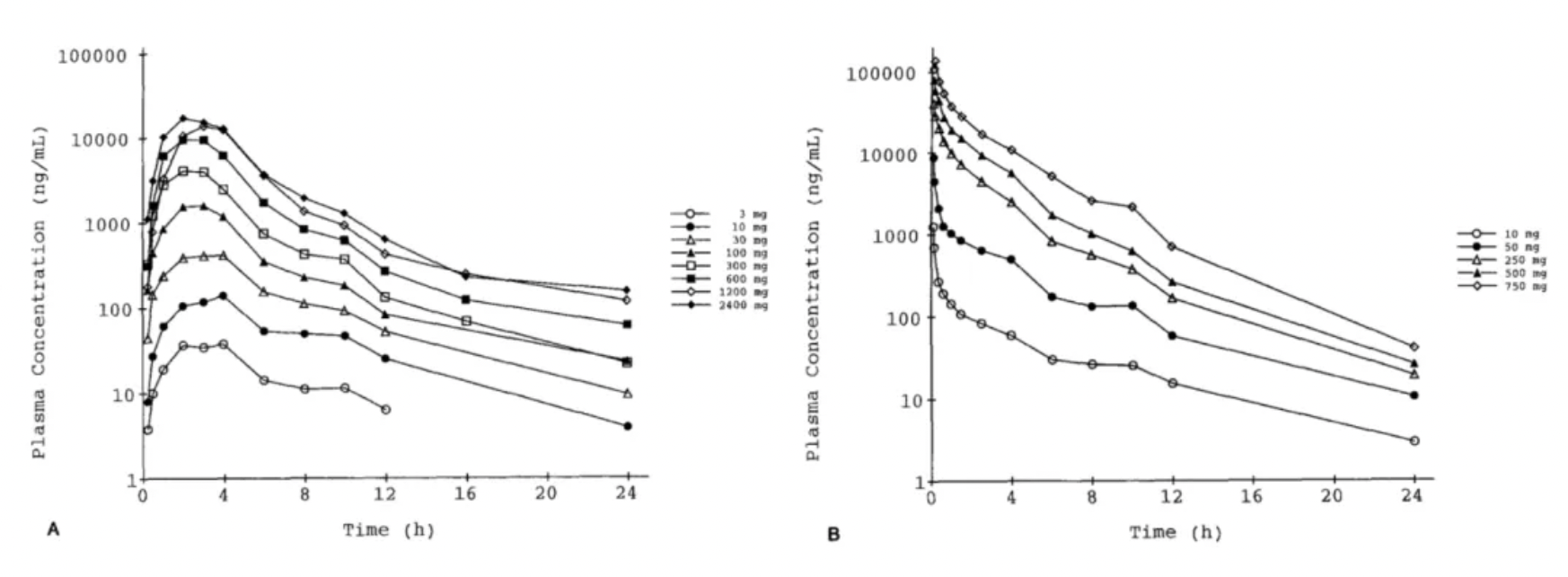
In 1996, the Roche team published the first study on the pharmacokinetics and pharmacodynamics of Bosentan in healthy humans in the journal "Clin Pharmacol Ther."
The results revealed that the systemic plasma clearance and the volume of distribution for Bosentan decreased with increasing doses, with approximate values of 6 L/h and 0.2 L/kg, respectively. The absolute bioavailability was around 50% and appeared to decrease with doses exceeding 600 mg. The maximal increase in plasma ET-1 was 2-fold (oral) and 3-fold (intravenous). Bosentan reversed the vasoconstrictive effects of ET-1 measured in the skin microcirculation, leading to a trend of decreasing blood pressure (by about 5 mm Hg) and an increase in heart rate (by about 5 beats/min). The oral administration of Bosentan was well tolerated, while higher doses administered intravenously caused vomiting and local intolerance.
PIVLAZ® (Clazosentan) is a potent and selective endothelin ETA receptor antagonist developed by Idorsia Pharmaceuticals. It is indicated for the prevention of cerebral vasospasm, vasospasm-related cerebral infarction, and cerebral ischemic symptoms following aneurysmal subarachnoid hemorrhage (aSAH). PIVLAZ® was successfully launched in Japan in April 2022 for the treatment of cerebral vasospasm. Sosei Heptares holds the marketing rights for PIVLAZ® in Japan and the Asia-Pacific region, excluding China.
It is reported that between 40% and 70% of patients experience cerebral vasospasm within 4 to 14 days after aneurysmal subarachnoid hemorrhage (aSAH). Of these patients with cerebral vasospasm, 17% to 40% develop delayed ischemic neurological deficits, with approximately half of them suffering from cerebral infarction. Post-acute cerebral infarction, endothelin levels are elevated under the induction of oxyhemoglobin and released from red blood cells. This leads to cerebral vasospasm through the endothelin ETA receptor.
According to a clinical study report published by the Idorsia team in 2022, in each study, 221 patients underwent random treatment, with 220 patients included in the full analysis set for the primary analysis (109 patients in the Clazosentan group and 111 patients in the placebo group). Compared with placebo, a 150 mg intravenous infusion of Clazosentan significantly reduced the incidence of vasospasm-related morbidity and all-cause mortality following aSAH.

Aprocitentan is a novel oral dual endothelin receptor antagonist being developed by Idorsia Pharmaceuticals that effectively inhibits the binding of ET-1 to both ETA and ETB receptors. It is reported that the potential for drug-drug interactions with Aprocitentan is low, and its mechanism of action is very well suited to the pathophysiology of resistant hypertension.
In November 2022, the team from Idorsia and Johnson & Johnson authored an article in The Lancet journal, reporting phase 3 clinical study data for aprocitentan in the treatment of patients with treatment-resistant hypertension. The results indicated that aprocitentan was well tolerated in patients with treatment-resistant hypertension, demonstrating superior blood pressure lowering effects compared to placebo at week 4, with sustained effectiveness at week 40.
Sparsentan (BMS-346567) was developed by the BMS research team in 2005 and is the first orally active therapeutic among its class. Sparsentan is a fusion of structural elements from the AT1R antagonist irbesartan and the ETAR antagonist biphenylsulfonamide. It exhibits high affinity for both receptors, with an affinity of 9.3 nM for ETAR and 0.8 nM for AT1R.
In May 2023, the esteemed medical journal The Lancet online published clinical research findings by Travere Therapeutics regarding Sparsentan in patients with IgA nephropathy. The study showed that, compared to the AT1R antagonist irbesartan alone, daily treatment with the dual-acting agent Sparsentan significantly reduced proteinuria in adult patients with IgA nephropathy.
ETA, as a classically validated scientific and clinical target, has led to numerous developmental projects for ET antagonists centered around ETA. In addition to standalone ETA antagonists, the combination of ETA with multiple targets, including ETA+ETB, ETA+NF-κB, ETA+AT1R, ETA+ETB+PDE5A, ETA+PDE5A, ETA+CD3, ETA+TGF-β, has become an important approach in the development of innovative clinical candidate compounds.
In the context of drug types, in addition to classic small molecule therapeutics, synthetic peptides, monoclonal antibodies, and bispecific antibodies have also become new directions for the development of clinical candidate drugs.
While Pfizer and MSD (Japan) have either terminated or seen no progress in their research on synthetic peptides targeting ETA, Gmax Biopharm, a Chinese company, has independently led the antibody development in this area. Several branch pipelines are at the preclinical or clinical development stages.
Given the historical data from the development of multi-target combinations with small molecule drugs targeting ETA, there is significant potential in developing bispecific antibodies that target ETA and a second therapeutic target.