FDA Approves GT Biopharma's IND Application for GTB-3650 in CD33+ Leukemia Therapy
GT Biopharma, Inc., a clinical-stage immuno-oncology firm dedicated to the creation of advanced therapies using its unique natural killer cell engager, the TriKE platform, has revealed that the FDA has approved its IND application for GTB-3650. This approval permits the company to initiate a Phase 1 clinical trial, which is expected to begin in the latter half of 2024.
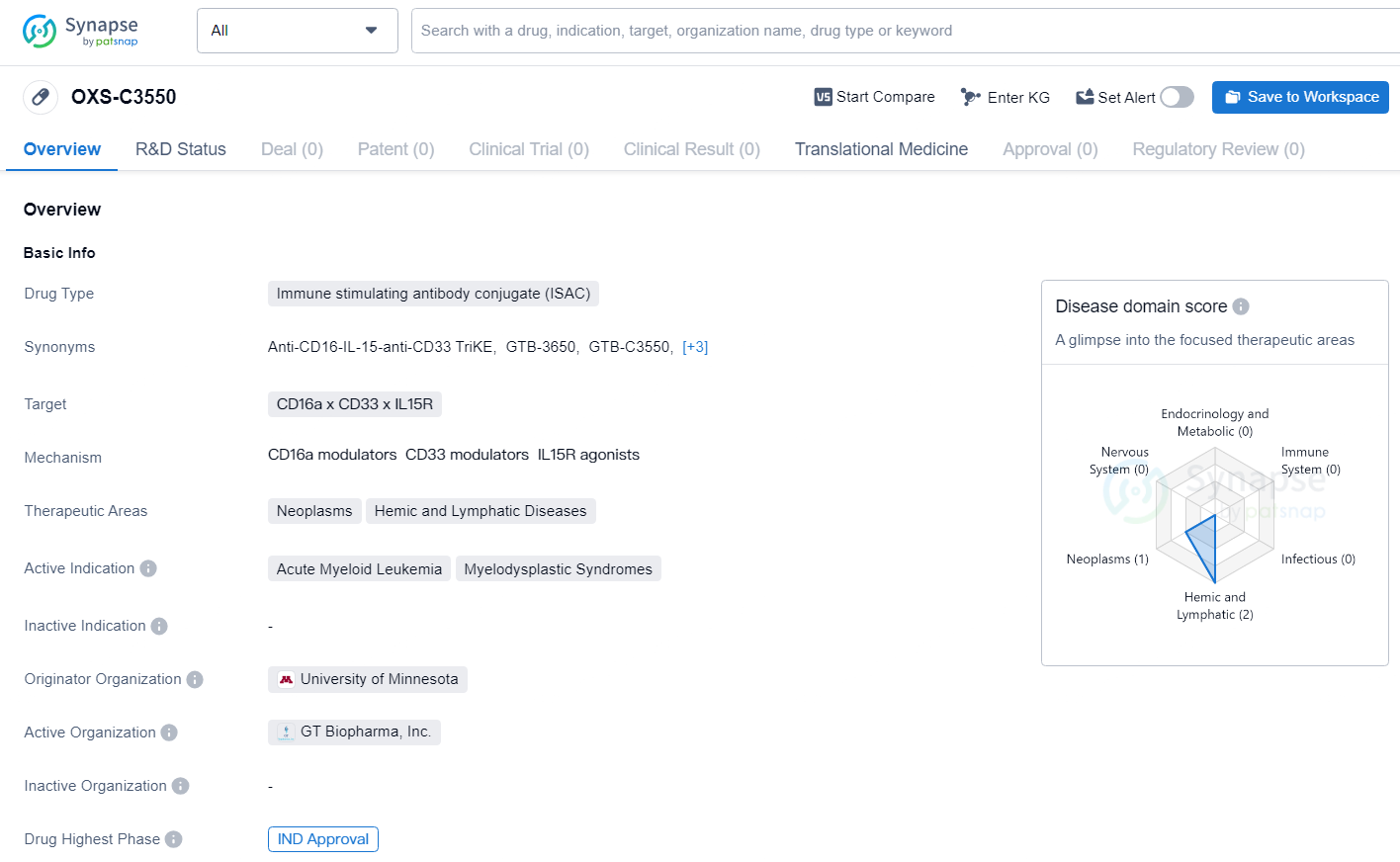
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
"The FDA clearance for GTB-3650 is a significant milestone, and we are eager to submit our next Investigational New Drug (IND) application in the first quarter of 2025 for GTB-5550, which aims to target multiple solid tumors," stated Michael Breen, Executive Chairman and Interim Chief Executive Officer of GT Biopharma.
"As we advance our clinical operations, we intend to initiate the Phase 1 trial for GTB-3650 within the next few months, with several data releases anticipated in 2025. Additionally, we plan to commence a basket trial using GTB-5550 for multiple solid tumors in 2025. We remain highly optimistic about exploring further prospects for various autoimmune conditions where our TriKEs could offer therapeutic benefits," Breen elaborated.
The Phase 1 dose-escalation study will assess GTB-3650 in up to six cohorts of adult patients with relapsed or refractory hematologic malignancies expressing CD33, such as acute myeloid leukemia and high-risk myelodysplastic syndrome. The dosing regimen for GTB-3650 will involve two-week cycles, administered for two weeks followed by a two-week break, extending up to four months based on observed clinical benefits. The trial aims to evaluate safety parameters, pharmacokinetics, pharmacodynamics, in vivo expansion of endogenous patient NK cells, and overall clinical activity.
"GTB-3650 is engineered to target NK cells in the immune system to potentially address the limitations of current AML chemotherapies," said Breen. "Our trial design is expected to provide initial insights into safety and possible therapeutic effects, as well as valuable information that will shape our clinical development plans for future TriKE molecules, including GTB-5550."
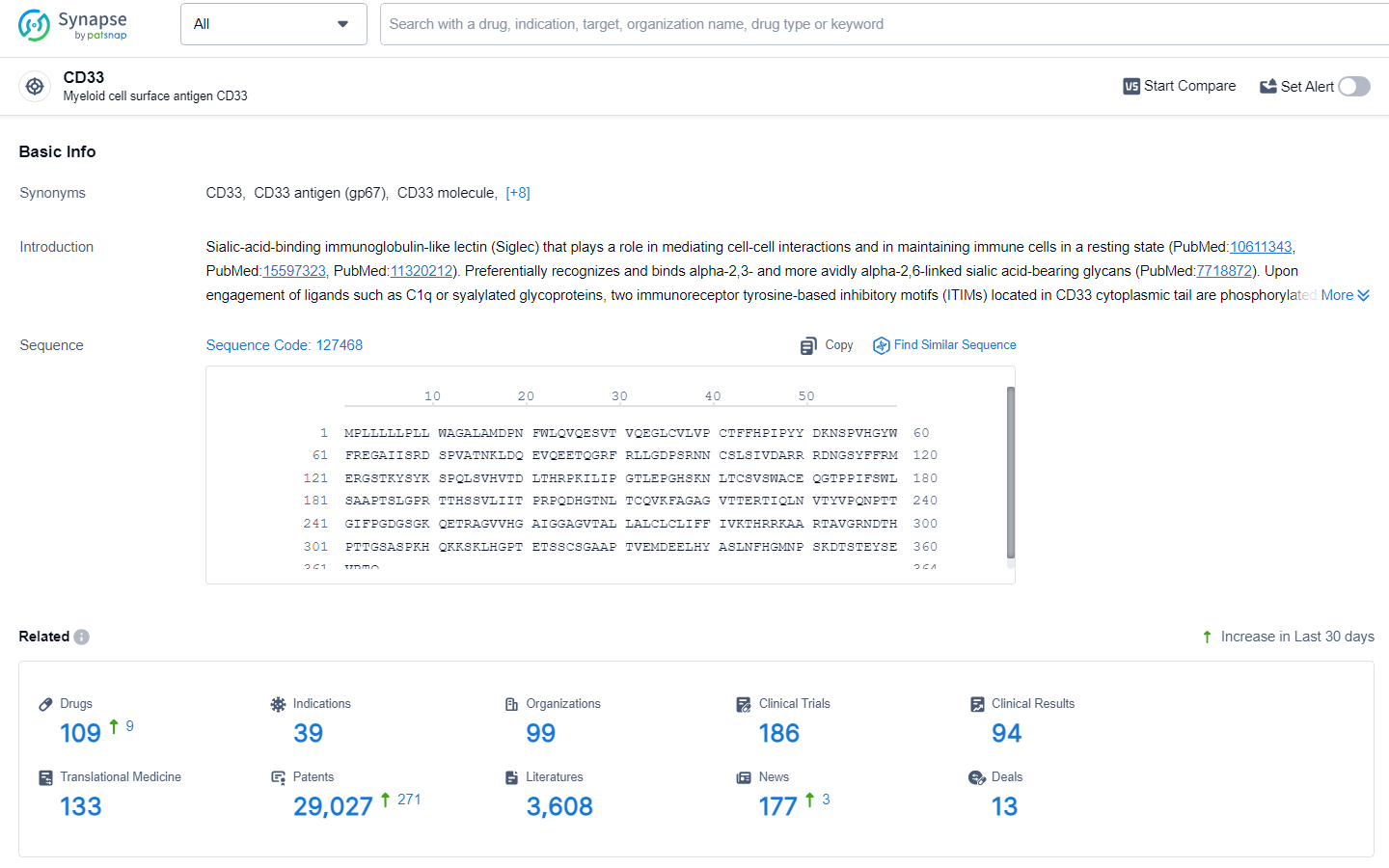
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of July 3, 2024, there are 109 investigational drugs for the CD33 target, including 39 indications, 99 R&D institutions involved, with related clinical trials reaching 186, and as many as 29027 patents.
OXS-C3550 is an immune stimulating antibody conjugate drug with a specific focus on targeting CD16a x CD33 x IL15R in the treatment of neoplasms, hemic, and lymphatic diseases, particularly acute myeloid leukemia and myelodysplastic syndromes. Developed by the University of Minnesota, the drug has received IND approval, marking an important step in its journey towards potential clinical use.