Initial Disease-Free Survival Results from Phase 1a ELI-002 7P AMPLIFY-7P Study by Elicio Therapeutics
Elicio Therapeutics, Inc., a biotechnology firm in the clinical stage focusing on innovative immunotherapies to combat cancer, has unveiled early findings from its current AMPLIFY-7P Phase 1a trial investigating the off-the-shelf therapeutic cancer vaccine candidate, ELI-002 7P. According to the preliminary results, patients administered the Phase 2 dose of 4.9mg AMP-peptide of ELI-002 7P had not achieved the median disease-free survival endpoint by the data cutoff date of May 24, 2024.
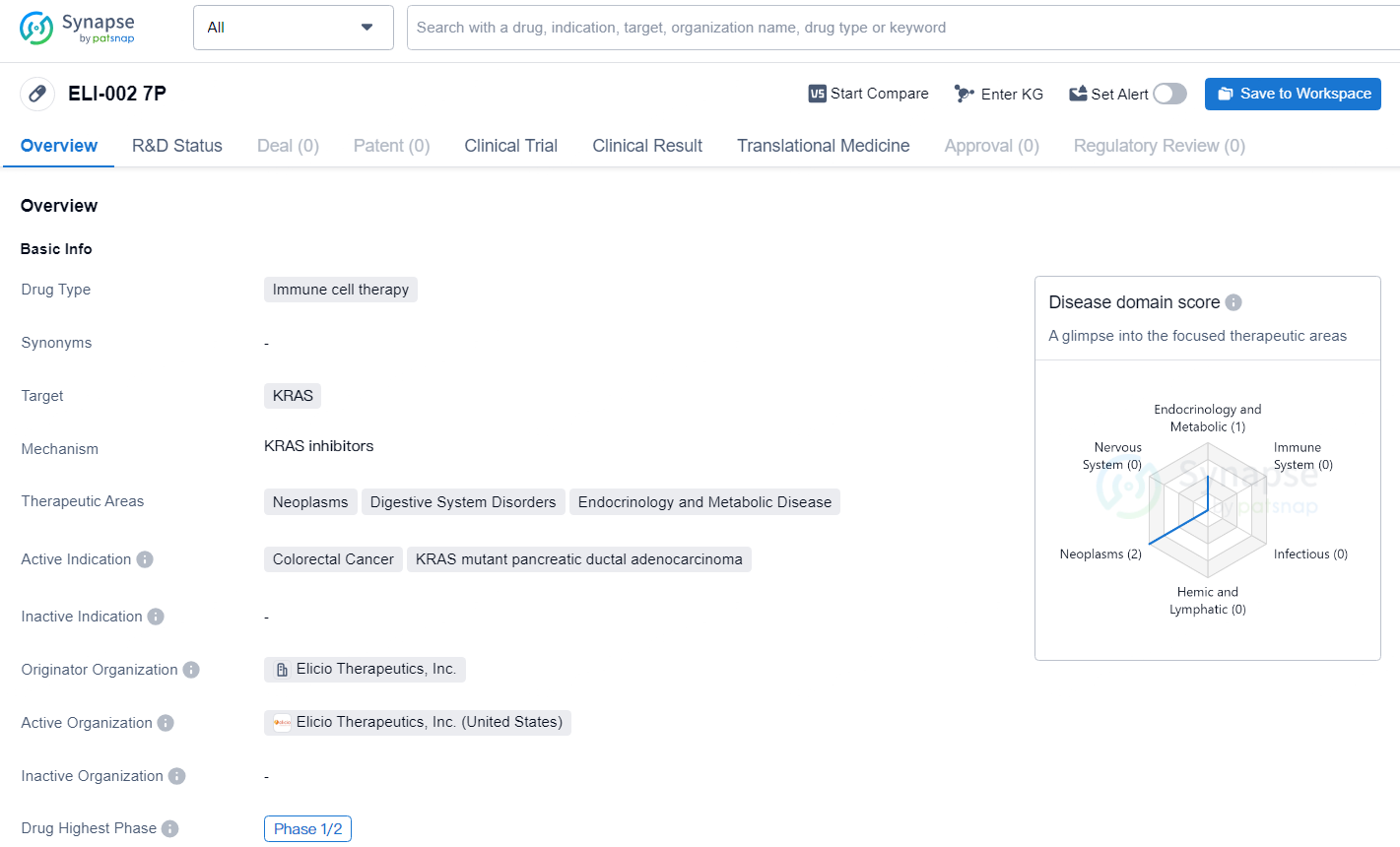
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The AMPLIFY-7P trial is assessing the efficacy of ELI-002 7P, a 7-peptide formulation, in individuals with mKRAS-driven solid tumors after they have received standard locoregional treatment. The data, as of May 24, 2024, focus on 14 patients with minimal residual disease who enrolled in the Phase 1 cohort of the AMPLIFY-7P study, with a median follow-up time for the DFS endpoint of 29.1 weeks.
Christopher Haqq, M.D., Ph.D., who serves as Elicio’s Executive Vice President, Head of Research and Development, and Chief Medical Officer, commented, “We are pleased to observe that patients treated with the 7-peptide formulation of ELI-002 at the Phase 2 dosage level continue to fare well at this preliminary stage of the Phase 1 trial. We anticipate sharing further clinical updates from the AMPLIFY Phase 1 trials later in 2024, along with the Phase 2 interim analysis results expected in the first quarter of 2025.”
Our primary product candidate, ELI-002, is an innovative experimental Amphiphile cancer vaccine targeting mKRAS gene mutations, a common factor in many cancers. ELI-002 is composed of two key elements built using our AMP technology: AMP-modified mutant KRAS peptide antigens and an AMP-modified CpG adjuvant, both designed for off-the-shelf subcutaneous administration.
Currently, ELI-002 2P is being tested in an ongoing Phase 1 trial involving patients with a high risk of relapse due to mKRAS-driven solid tumors, following surgery and chemotherapy. Meanwhile, ELI-002 7P is under investigation in a Phase 1/2 trial targeting patients with mKRAS-driven pancreatic cancer. The ELI-002 7P formulation aims to generate an immune response against seven prevalent KRAS mutations that are found in 25% of all solid tumors, thereby expanding the potential patient base for ELI-002.
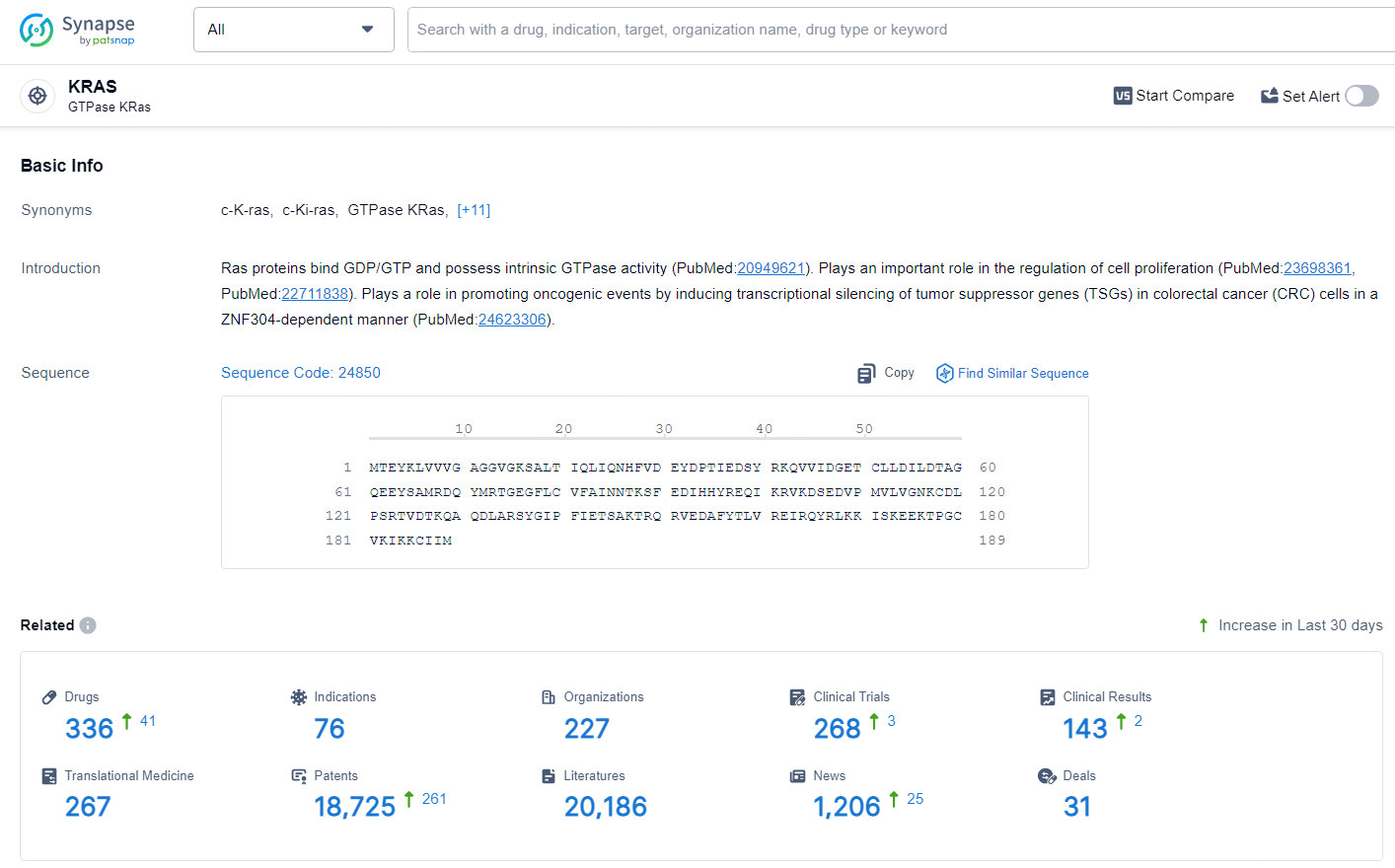
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of July 3, 2024, there are 336 investigational drugs for the KRAS target, including 76 indications, 227 R&D institutions involved, with related clinical trials reaching 268, and as many as 18725 patents.
ELI-002 7P represents an important advancement in the field of biomedicine, particularly in the area of immune cell therapy for cancer treatment. The drug's specific targeting of KRAS and its potential impact across multiple therapeutic areas underscore its significance in addressing unmet medical needs in cancer and related diseases.