FDA Approves HuidaGene's HG202, First CRISPR/Cas13 Treatment for Macular Degeneration
HuidaGene Therapeutics (“HuidaGene”), a biotechnology firm in the clinical development phase, has disclosed that the U.S. FDA has approved its investigational new drug (IND) application for HG202. This therapy is the inaugural CRISPR/Cas13 RNA-editing treatment to be utilized in clinical settings for the management of neovascular age-related macular degeneration (nAMD), a condition that impacts millions of people globally.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"The approval of an open IND for HG202 by the U.S. FDA—the first authority to authorize CRISPR/Cas13 for clinical applications—represents a crucial advancement for HuidaGene and the CRISPR RNA-editing arena,” stated Alvin Luk, Ph.D., M.B.A., C.C.R.A., Co-founder and CEO of HuidaGene. “Robust preclinical results for HG202, along with encouraging data from the initial human trial ‘SIGHT-I’ conducted in China, highlight its potential to treat AMD via a non-receptor binding mechanism, utilizing the Cas13 RNA editor to reduce VEGF-A mRNA levels.”
Xin Zhang, M.D., MSc., COO and CMO of HuidaGene, emphasized the need for novel treatments for nAMD: “As many as 46% of AMD patients exhibit inadequate responses or develop resistance to anti-VEGF treatments. These patients require safe and effective alternatives. The BRIGHT trial aims to assess the safety and efficacy of HG202 to fill this void, and we are eager to begin patient enrollment soon.”
Hui Yang, Ph.D., Co-Founder and Chief Scientific Advisor of HuidaGene, remarked, “Our AI/ML-based HG-PRECISE® platform enabled us to identify the Cas13X/Y system (Nature Methods 2021). Building from this foundation, my team successfully engineered a high-fidelity Cas13Y that offers efficient editing with minimal off-target impacts (Nature Biotechnology 2022), establishing the technical groundwork for future clinical uses.”
BRIGHT Trial of HG202 (NCT06623279)
The BRIGHT trial, an open-label, dose-escalation Phase 1 study, will explore the safety and efficacy of HG202 across various doses in patients with neovascular AMD. The primary outcomes will focus on the safety and tolerability of HG202, while secondary outcomes will evaluate enhancements in visual acuity, retinal thickness, and reduction in the necessity for anti-VEGF injections.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
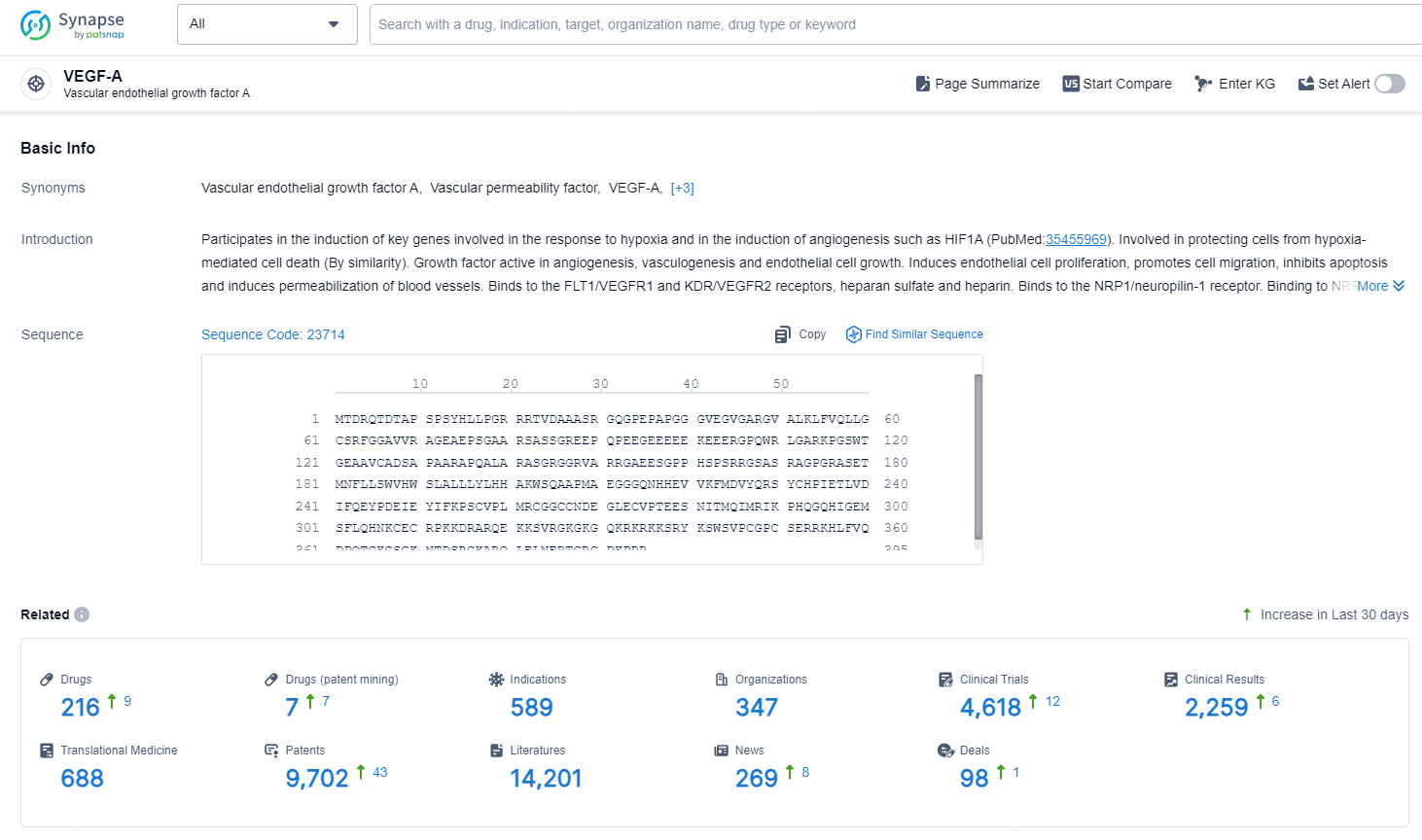
According to the data provided by the Synapse Chemical, As of November 5, 2024, there are 216 investigational drugs for the VEGF-A target, including 589 indications, 347 R&D institutions involved, with related clinical trials reaching 4618, and as many as 9702 patents.
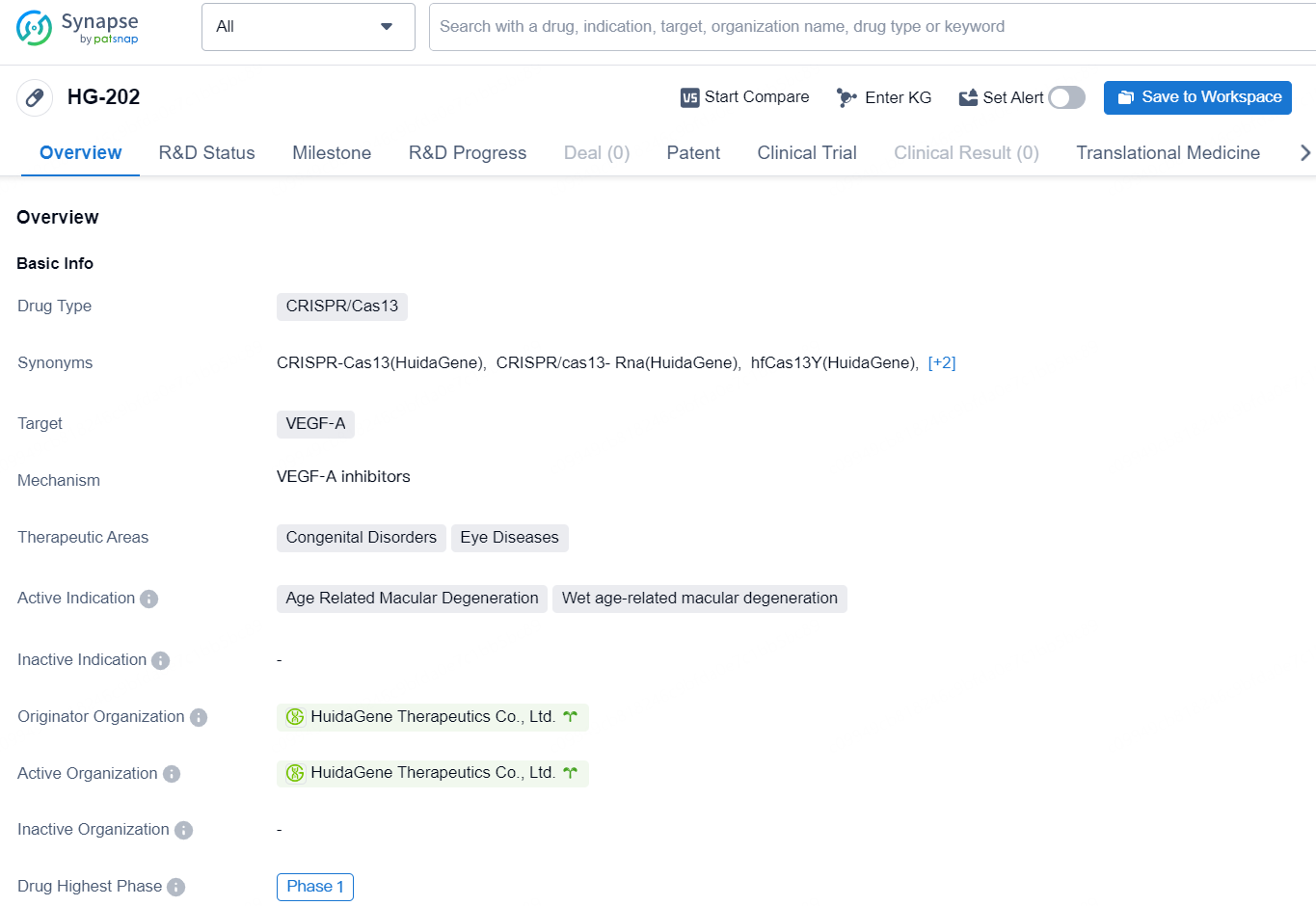
The drug HG-202 is a CRISPR/Cas13 type drug developed by HuidaGene Therapeutics Co., Ltd. The drug targets Vascular Endothelial Growth Factor A (VEGF-A) and has therapeutic applications in Congenital Disorders and Eye Diseases. The active indications for [HG-202] include Age Related Macular Degeneration and Wet age-related macular degeneration.