FDA Approves KaliVir Immunotherapeutics' VET3-TGI for Solid Tumor Immunotherapy
KaliVir Immunotherapeutics, Inc., a biotechnology firm focused on creating advanced, multi-mechanistic oncolytic viral immunotherapy programs, has reported that the FDA has approved the Investigational New Drug application for the STEALTH-001 trial of VET3-TGI in patients with terminal, advanced solid tumors.
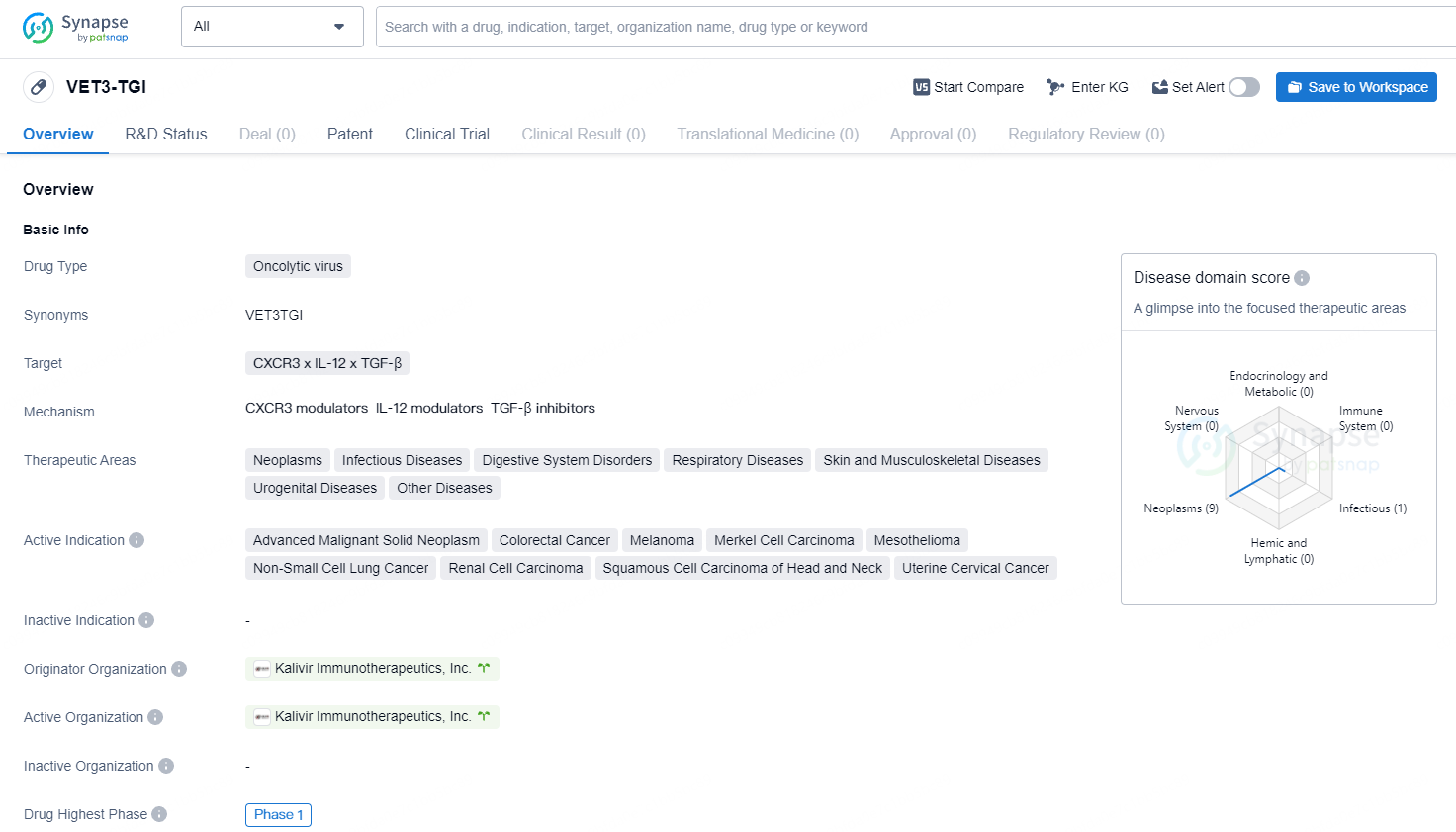
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
VET3-TGI represents an innovative form of oncolytic immunotherapy, designed to selectively target and destroy tumor cells while also enhancing anti-cancer immune responses through the expression of interleukin-12 and a TGFbeta inhibitor. Nonclinical studies have demonstrated its ability to effectively kill cancer cells while promoting immune system activation.
The Phase 1/1b clinical trial is set to investigate the safety and effectiveness of VET3-TGI, administered either via intravenous infusion or directly into the tumor, in patients suffering from advanced, inoperable solid tumors. This study will examine VET3-TGI as a standalone treatment as well as in conjunction with checkpoint inhibitor therapy.
"The launch of this Phase 1/1b clinical trial signifies a crucial step in our mission to revolutionize cancer therapy using oncolytic virus approaches, particularly for advanced, unmanageable, or metastatic solid tumors," commented Helena Chaye, Ph.D., CEO of KaliVir Immunotherapeutics.
"This is our second clinical trial initiation under the VET platform, following the 2023 progress of ASP1012, which is exclusively licensed to Astellas. We are dedicated to expanding the possibilities of cancer treatment, developing safer and more effective therapeutic options that could potentially reshape the oncology treatment landscape," added Helena Chaye.
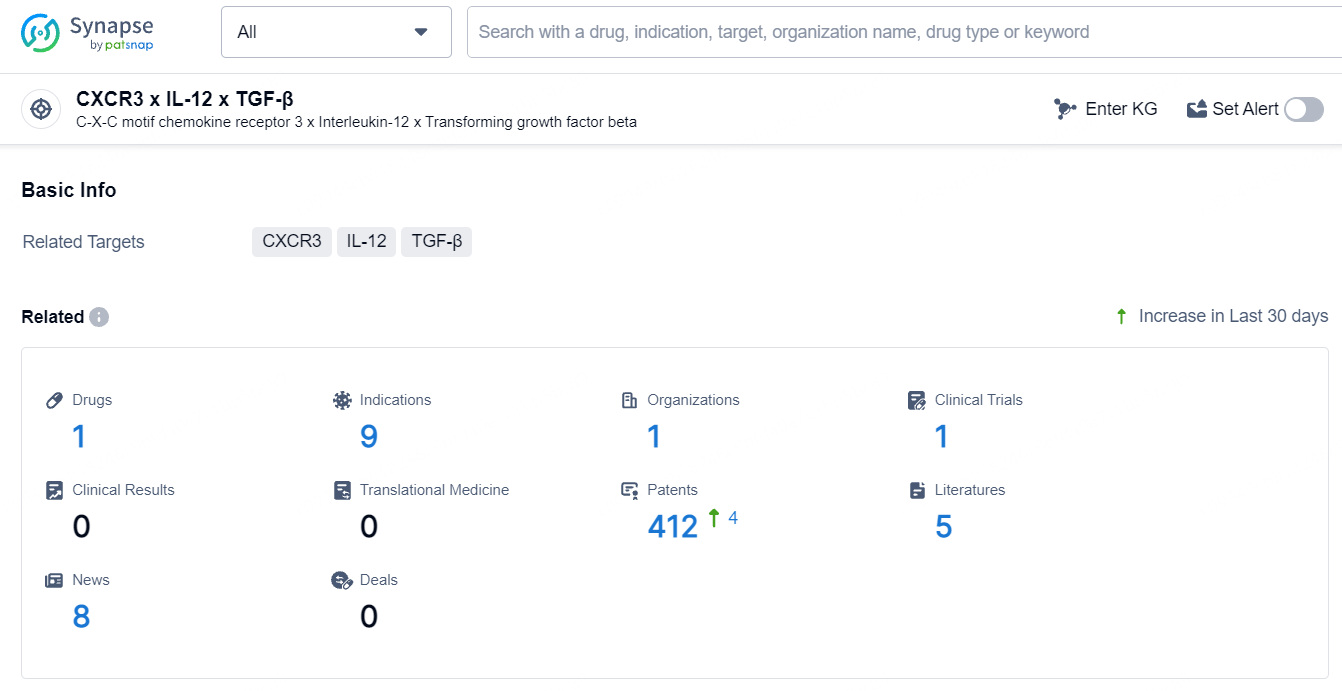
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of July 5, 2024, there are 1 investigational drug for the CXCR3, IL-12, and TGF-β targets, including 9 indications, 1 R&D institution involved, with related clinical trial reaching 1, and as many as 412 patents.
VET3-TGI is an oncolytic virus that targets CXCR3, IL-12, and TGF-β. VET3-TGI is an oncolytic virus with a broad range of therapeutic areas and active indications, particularly focusing on various types of cancer.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!