Idorsia's JERAYGO: Europe's First and Only ERA Approved for Resistant Hypertension
Idorsia Ltd stated that JERAYGO™ (aprocitentan) has received approval from the European Commission for use in adult patients with resistant hypertension, in conjunction with a minimum of three antihypertensive medications. The suggested dosage is 12.5 mg taken orally once each day. For patients who can tolerate the 12.5 mg dosage and require more stringent blood pressure management, the dose may be raised to 25 mg taken once daily.
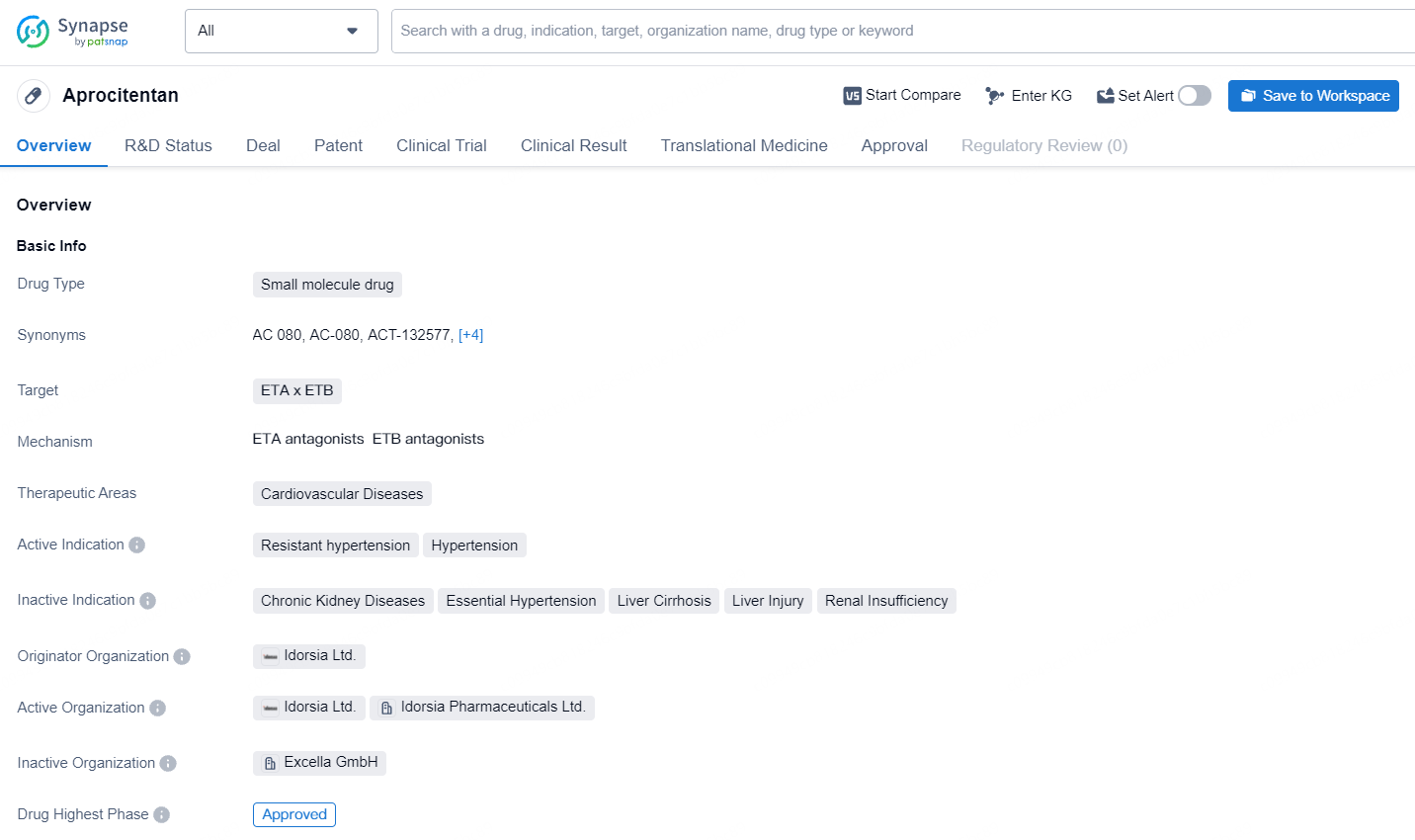
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
High blood pressure is a major contributor to cardiovascular illness globally, affecting roughly 1.3 billion individuals. Around 10% of these individuals have hypertension that is not controlled, even though they are taking at least three different types of antihypertensive medications at recommended doses. These patients are identified in hypertension guidelines as having resistant hypertension.
Professor Krzysztof Narkiewicz, MD, PhD, leader of the Department of Hypertension and Diabetology at the Medical University of Gdansk, Poland, stated: “JERAYGO is an oral antihypertensive treatment targeting a novel therapeutic pathway – the endothelin system. JERAYGO has shown significant effectiveness in swiftly and sustainably lowering blood pressure. With JERAYGO, physicians now have a potent new treatment option for managing blood pressure in these patients.”
Alberto Gimona, MD, Head of Global Clinical Development & Medical Affairs, commented: “We are extremely proud to have secured approval for JERAYGO, the first new anti-hypertensive drug in four decades targeting the endothelin pathway, which we believe plays a crucial role in patients with resistant hypertension. Additionally, JERAYGO demonstrated a notable drop in albuminuria, as indicated by a reduction in baseline UACR. I am delighted that the comprehensive data we have collected on JERAYGO is accurately reflected in its labeling. We are now working to extend marketing authorization, seeking approval for JERAYGO in the UK, Canada, and Switzerland.”
André Muller, CEO of Idorsia, remarked: “With aprocitentan, we have a significantly valuable asset approved in both the US and Europe. We are continuing to thoroughly assess all funding avenues, including possible collaborations for the commercialization of aprocitentan, as we prepare to launch aprocitentan in these two critical markets.”
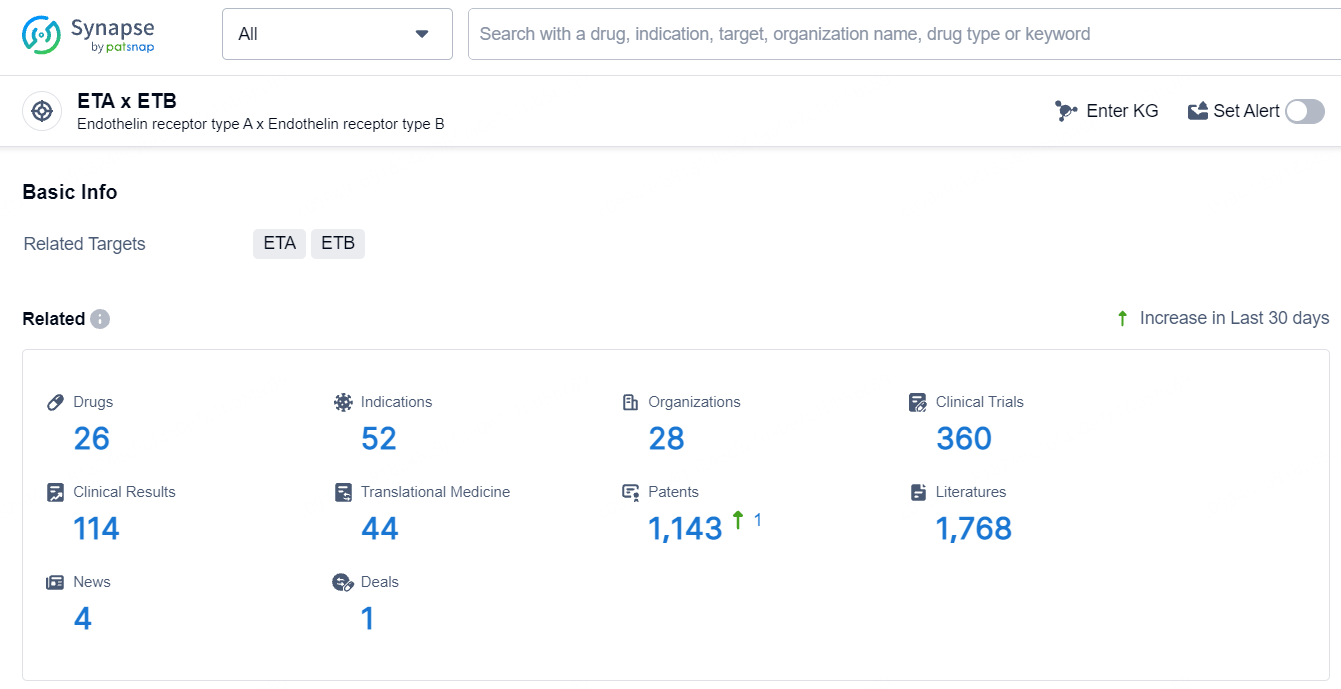
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of July 5, 2024, there are 26 investigational drugs for the ETA and ETB target, including 52 indications, 28 R&D institutions involved, with related clinical trials reaching 360, and as many as 1143 patents.
Aprocitentan is a small molecule drug developed by Idorsia Ltd., targeting the ETA and ETB receptors. Aprocitentan represents a promising development in the field of biomedicine, offering a new treatment option for patients with resistant hypertension and hypertension. Its pending approval in China indicates the potential for global availability, and its approval in the United States in 2024 will mark a significant milestone in its journey to market.