FDA Approves Kymera Therapeutics' IND Application for Novel Oral STAT6 Degrader KT-621
Kymera Therapeutics, Inc. (NASDAQ: KYMR), a biopharmaceutical firm in the clinical stage that is developing a novel category of small molecule therapeutics through targeted protein degradation (TPD), has revealed that its Investigational New Drug (IND) submission has been approved by the U.S. Food and Drug Administration (FDA) for KT-621, a strong and selective oral STAT6 degrader. The company plans to begin administration in a Phase 1 clinical trial involving healthy participants in October 2024, with data from this trial expected to be released in the first half of 2025.
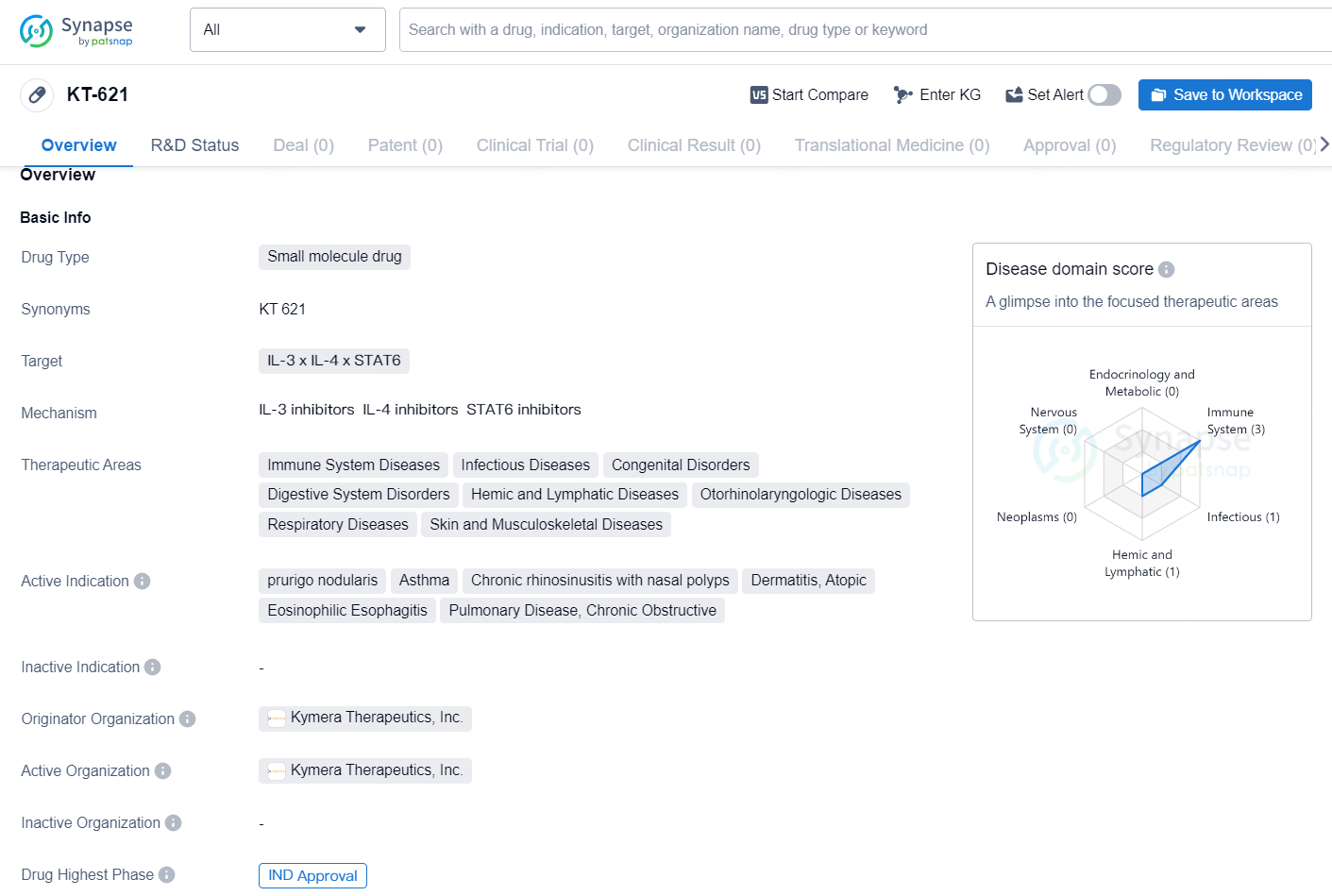
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
"The FDA granting clearance for the KT-621 IND marks a pivotal achievement for Kymera, its patients, and the broader industry, enabling Kymera to be the first organization to move a STAT6-targeted therapy into clinical trials," stated Nello Mainolfi, PhD, Founder, President, and CEO of Kymera Therapeutics. "Our oral STAT6 degrader, KT-621, is unlike conventional oral small molecule inhibitors; we believe it has the potential to integrate the complete pathway blockade provided by upstream biologics with the ease of oral delivery. This innovative approach may revolutionize the current treatment options for atopic and allergic diseases. We are eager to proceed with KT-621 into Phase 1 clinical trials and look forward to providing updates on this initiative soon."
The Phase 1 study aims to assess the safety, tolerability, pharmacokinetics, and pharmacodynamics of both single and multiple ascending doses of KT-621 in comparison to a placebo.
STAT6 has historically been an unaddressed key transcription factor within the IL-4/IL-13 signaling pathways and acts as the main driver of T helper type 2 (TH2) inflammation in allergic conditions. Several gain-of-function mutations in STAT6 have been linked to severe allergic disorders in humans. Dupilumab, a monoclonal antibody administered via injection to inhibit IL-4/IL-13 signaling, is an approved treatment for various allergic and atopic conditions.
This indicates strong support for targeting STAT6, based on both genetic findings and clinical validation of the pathway. STAT6 operates through interactions between proteins and DNA, which has made it difficult to effectively inhibit it using small molecule inhibitors. Nonetheless, we believe that a targeted protein degradation strategy is appropriate for this purpose, as even a single binding event can trigger degradation. KT-621 is an investigational first-in-class oral STAT6 degrader that is taken once daily and exhibits activity similar to dupilumab in preclinical studies. It has the potential to tackle various allergic and atopic diseases, such as atopic dermatitis, asthma, and chronic obstructive pulmonary disease, among others. Kymera plans to start Phase 1 trials for KT-621 in October 2024, with initial data expected in the first half of 2025.
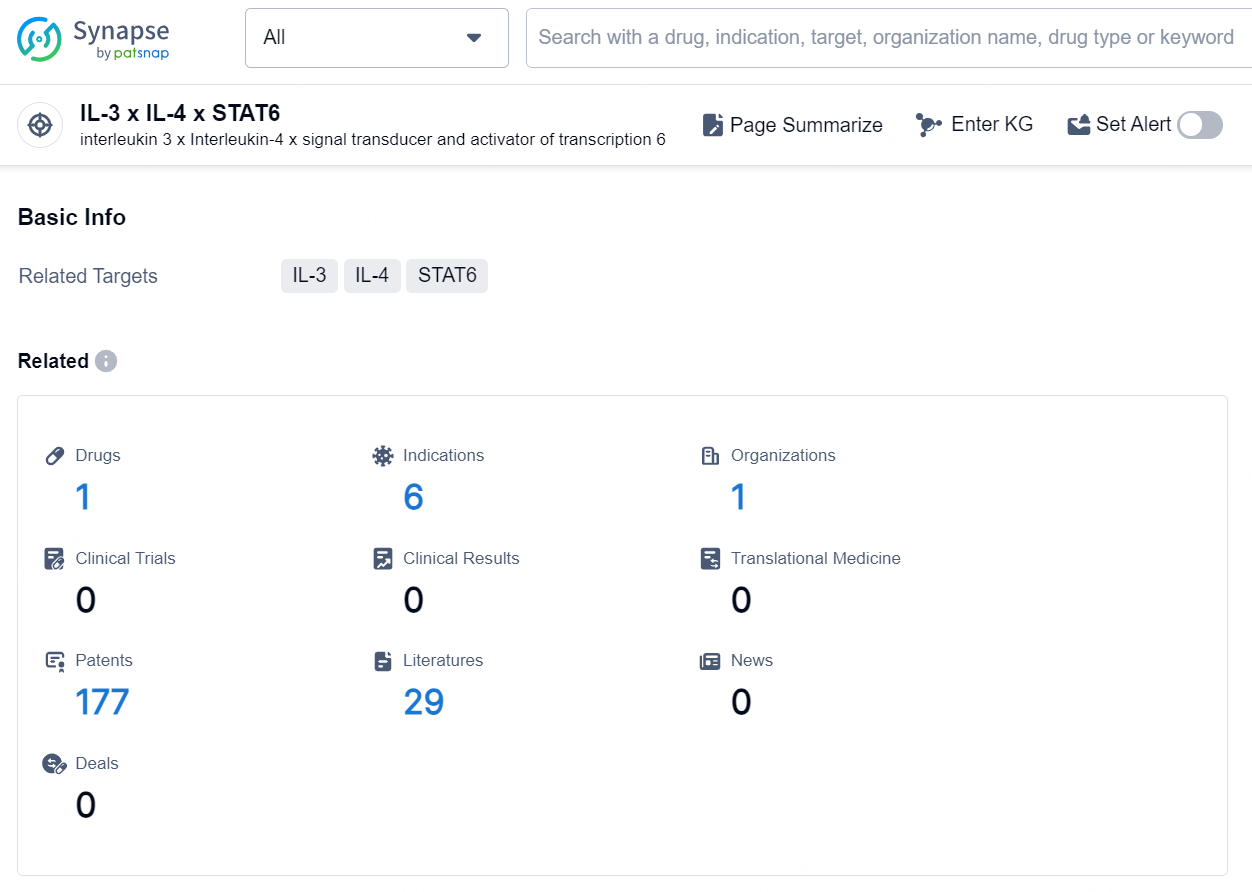
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of October 10, 2024, there are 1 investigational drug for the IL-3 x IL-4 x STAT6 target, including 6 indications, 1 R&D institution involved, and as many as 177 patents.
The drug KT-621 is a small molecule drug that targets IL-3, IL-4, and STAT6. It has potential therapeutic applications in various areas, including immune system diseases, infectious diseases, congenital disorders, digestive system disorders, hemic and lymphatic diseases, otorhinolaryngologic diseases, respiratory diseases, and skin and musculoskeletal diseases.