The FDA has received a New Drug Application for elinzanetant
Bayer has revealed that the U.S. Food and Drug Administration (FDA) has accepted the company’s New Drug Application (NDA) for the investigational drug elinzanetant, which is aimed at obtaining approval for managing moderate to severe vasomotor symptoms (VMS, commonly referred to as hot flashes) linked to menopause.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"The acceptance of elinzanetant's NDA by the FDA represents a crucial step forward in our mission to enhance menopause management for women in the U.S.," stated Christine Roth, Executive Vice President of Global Product Strategy and Commercialization and a member of Bayer's Pharmaceutical Leadership Team. "If it receives approval, elinzanetant will provide a new non-hormonal treatment option for women experiencing moderate to severe vasomotor symptoms, and we eagerly anticipate the agency's review."
The NDA submission is supported by favorable outcomes from the OASIS 1, 2, and 3 Phase III trials, which assessed the safety and effectiveness of the investigational treatment elinzanetant compared to placebo. Results from both OASIS 1 and 2 were published in JAMA in August 2024. Comprehensive data from the OASIS 3 Phase III trial, which includes additional long-term safety and efficacy information, were shared during The Menopause Society's annual meeting in September 2024.
Bayer is also actively pursuing marketing authorizations for elinzanetant with other regulatory bodies.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
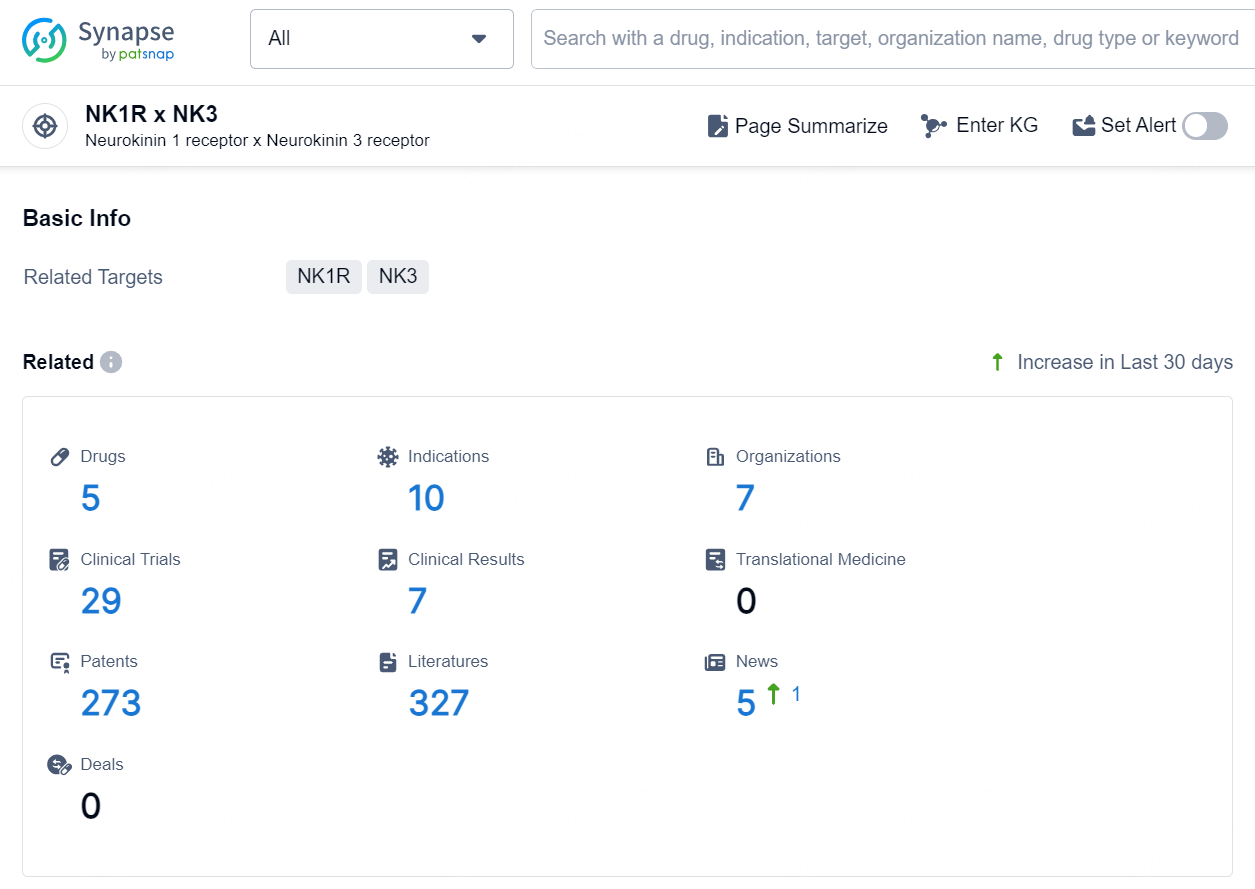
According to the data provided by the Synapse Database, As of October 11, 2024, there are 5 investigational drugs for the NK1R x NK3 targets, including 10 indications, 7 R&D institutions involved, with related clinical trials reaching 29, and as many as 273 patents.
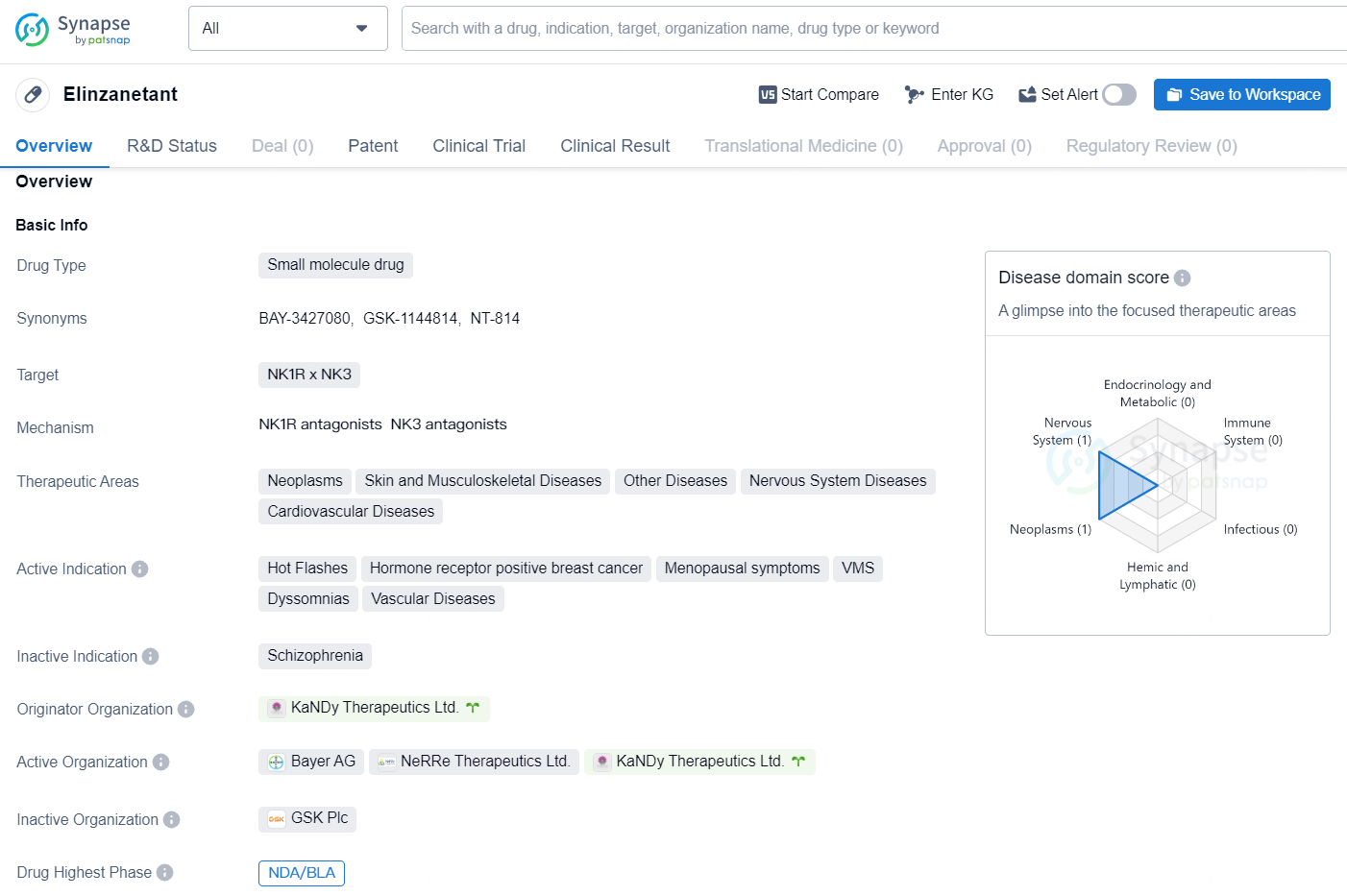
Elinzanetant is a small molecule drug that targets the Neurokinin-1 receptor (NK1R) and the Neurokinin-3 receptor (NK3). It has shown potential therapeutic benefits in a wide range of areas including neoplasms, skin and musculoskeletal diseases, nervous system diseases, cardiovascular diseases, and other diseases. The drug is being developed to address hot flashes, hormone receptor positive breast cancer, menopausal symptoms, vasomotor symptoms (VMS), dyssomnias, and vascular diseases. The drug is developed by KaNDy Therapeutics Ltd. and has reached the highest phase of New Drug Application (NDA) or Biologics License Application (BLA) on a global scale.