FDA Approves Mirum's LIVMARLI for Itchy Skin in Liver Disorder Patients
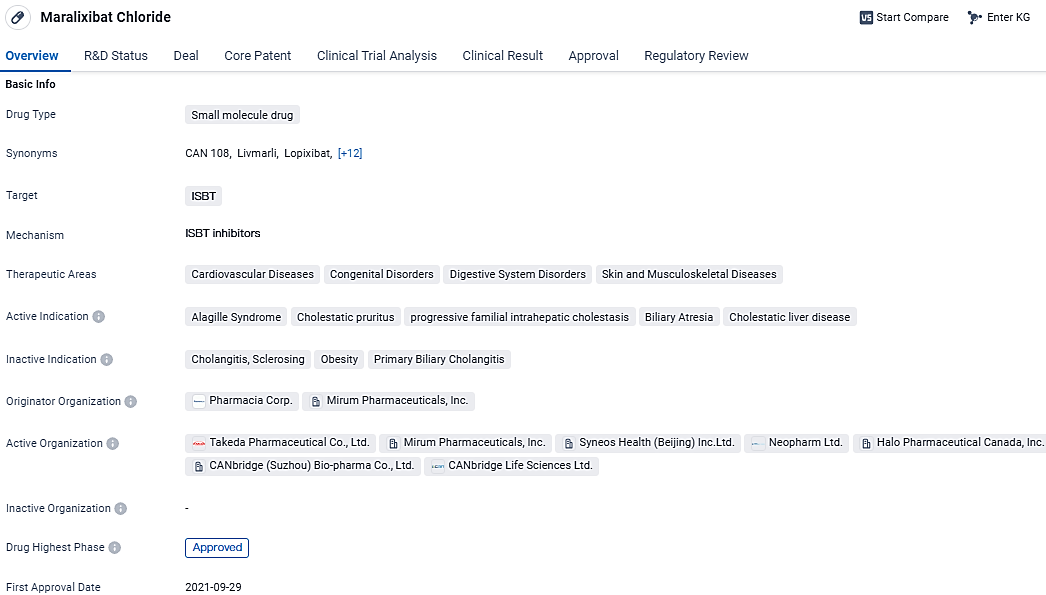
Mirum Pharmaceuticals, Inc. has revealed that LIVMARLI® (maralixibat), an oral medication, has received the green light from the U.S. Food and Drug Administration for use in alleviating itching caused by cholestasis in individuals aged five and above who are diagnosed with progressive familial intraheatic cholestasis.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Mirum has recently made a request to health authorities for an amended authorization to market a more concentrated version of LIVMARLI, which was utilized in the MARCH trial. The aim is to broaden the drug's approved use to include treatment for younger individuals suffering from PFIC later in the year. LIVMARLI is also sanctioned for managing Alagille syndrome-related cholestatic itch in the United States, Europe, Canada, and several other countries worldwide.
"LIVMARLI could markedly change the treatment landscape for individuals suffering from the intense itching linked with PFIC and is particularly important for those with exceptionally rare subtypes," commented Chris Peetz, Mirum’s CEO. “We are grateful to the patients, their families, and medical professionals whose participation in studies enabled this drug’s sanctioning.”
The grant of the drug’s expanded use draws upon findings from the Phase 3 MARCH trial, which stands as the most extensive controlled study ever carried out for PFIC. It included 93 individuals with various genetic forms of PFIC, such as PFIC1, PFIC2, PFIC3, PFIC4, PFIC6, and some with unclassified genetic alterations.
"The endorsement of LIVMARLI for treating cholestatic pruritus in PFIC patients comes after extensive research efforts that compiled a robust set of clinical data. This data demonstrated significant improvements in several key health markers, including the severe itching that afflicts children with PFIC," expressed Richard Thompson, a molecular hepatology professor at King’s College London and one of the MARCH study's researchers.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
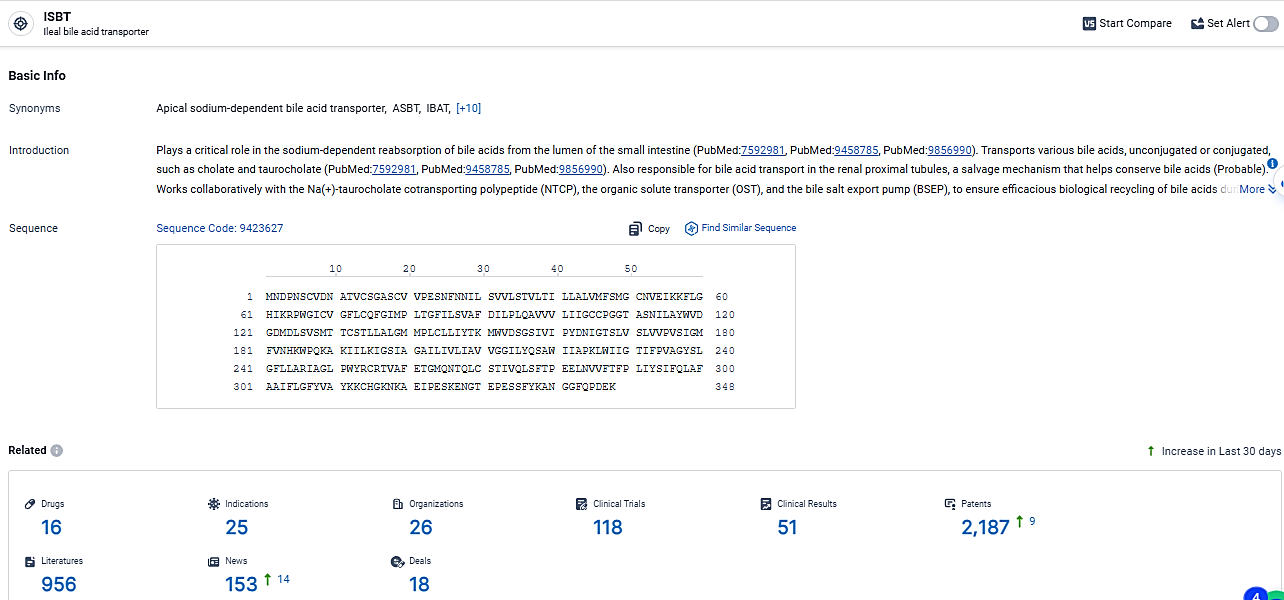
According to the data provided by the Synapse Database, As of March 17, 2024, there are 16 investigational drugs for the ISBT target, including 25 indications, 26 R&D institutions involved, with related clinical trials reaching 118, and as many as 2187 patents.
LIVMARLI® (maralixibat) oral solution is an orally administered, once-daily, ileal bile acid transporter inhibitor and the only approved medication by the U.S. Food and Drug Administration for the treatment of cholestatic pruritus in patients with Alagille syndrome three months of age and older and progressive familial intrahepatic cholestasis five years of age and older. Its approval marks a significant advancement in the treatment options available for patients with conditions related to impaired bile flow.