FDA Approves NMD Pharma's Phase 2 Trial of NMD670 for Charcot-Marie-Tooth Disease in US
NMD Pharma A/S, a biotech firm at the clinical stage focused on creating innovative and enhanced therapies for individuals suffering from serious neuromuscular disorders, announced today that the US Food and Drug Administration has approved its Investigational New Drug application. This approval paves the way for the initiation of a Phase 2 clinical trial, named SYNAPSE-CMT, examining NMD670 in patients with Charcot-Marie-Tooth type 1 and type 2 diseases. NMD670 is the first-in-its-class small molecule inhibitor targeted at muscles and specifically inhibits the ClC-1 chloride ion channel in skeletal muscles.
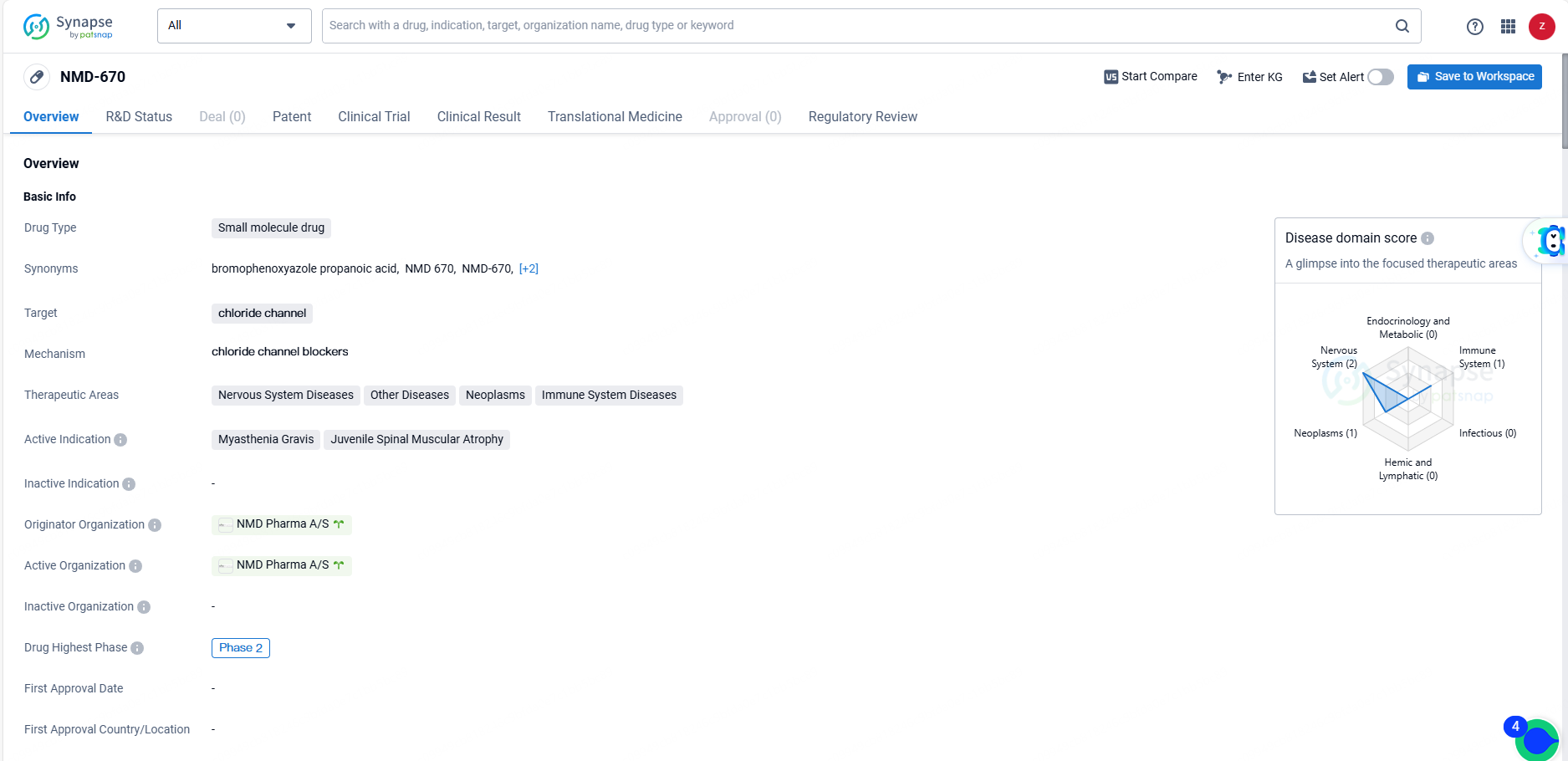
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The Phase 2 clinical trial will be a randomized, double-blind, placebo-controlled study designed to assess the efficacy, safety, and tolerability of NMD670 when administered orally twice daily over a 21-day period in around 80 adult patients with any genetically confirmed subtype of CMT1 or CMT2. Key endpoints of the trial include the 6-minute walk test, the 10-meter walk/run test, and the timed-up-and-go test, among other measures. The trial is set to be conducted at clinical sites in the United States and Europe, with patient enrollment expected to commence shortly.
In June 2023, NMD Pharma disclosed the outcomes of ESTABLISH1, an international observational study examining neuromuscular junction function in CMT types 1 and 2, at the Peripheral Nerve Society Annual Meeting. The findings indicated that dysfunction at the neuromuscular junction is a previously underappreciated characteristic in patients with the hereditary neurological disorders CMT types 1 and 2. The study showed that in CMT patients, nerves undergoing degeneration transmit signals to skeletal muscle with varying degrees of weakness and deficits.
The level of transmission deficit at the neuromuscular junction in CMT patients was found to correlate with disease severity and motor function as evaluated by various clinical measures of muscle strength and function.
Thomas Holm Pedersen, CEO of NMD Pharma, stated: “Charcot-Marie-Tooth disease is a severely debilitating condition with no cure or approved treatments available. There is an urgent need to develop therapies. The results from the ESTABLISH study highlight that neuromuscular junction transmission deficits are a previously unrecognized feature of CMT. This gives us confidence that NMD670 has the potential to work within skeletal muscles to provide and sustain clinically significant benefits for this underserved patient group.”
Allison Moore, CEO of the Hereditary Neuropathy Foundation, commented: “NMD Pharma’s focus on developing treatments that target the symptoms of various CMT subtypes is a crucial step toward significantly improving the lives of patients. By addressing the daily challenges of this debilitating condition and potentially restoring function, NMD Pharma offers hope and tangible benefits to those in need.”
By the end of 2024, NMD Pharma will have three ongoing Phase 2 trials employing its skeletal muscle activation enhancing therapy in rare neuromuscular diseases with significant patient impact and unmet need. These include a Phase 2 study of NMD670 for adults with spinal muscular atrophy type 3, a Phase 2b study of NMD670 in generalized myasthenia gravis, both of which have already begun, and following today’s announcement, a Phase 2 study of NMD670 in patients with CMT type 1 and type 2.
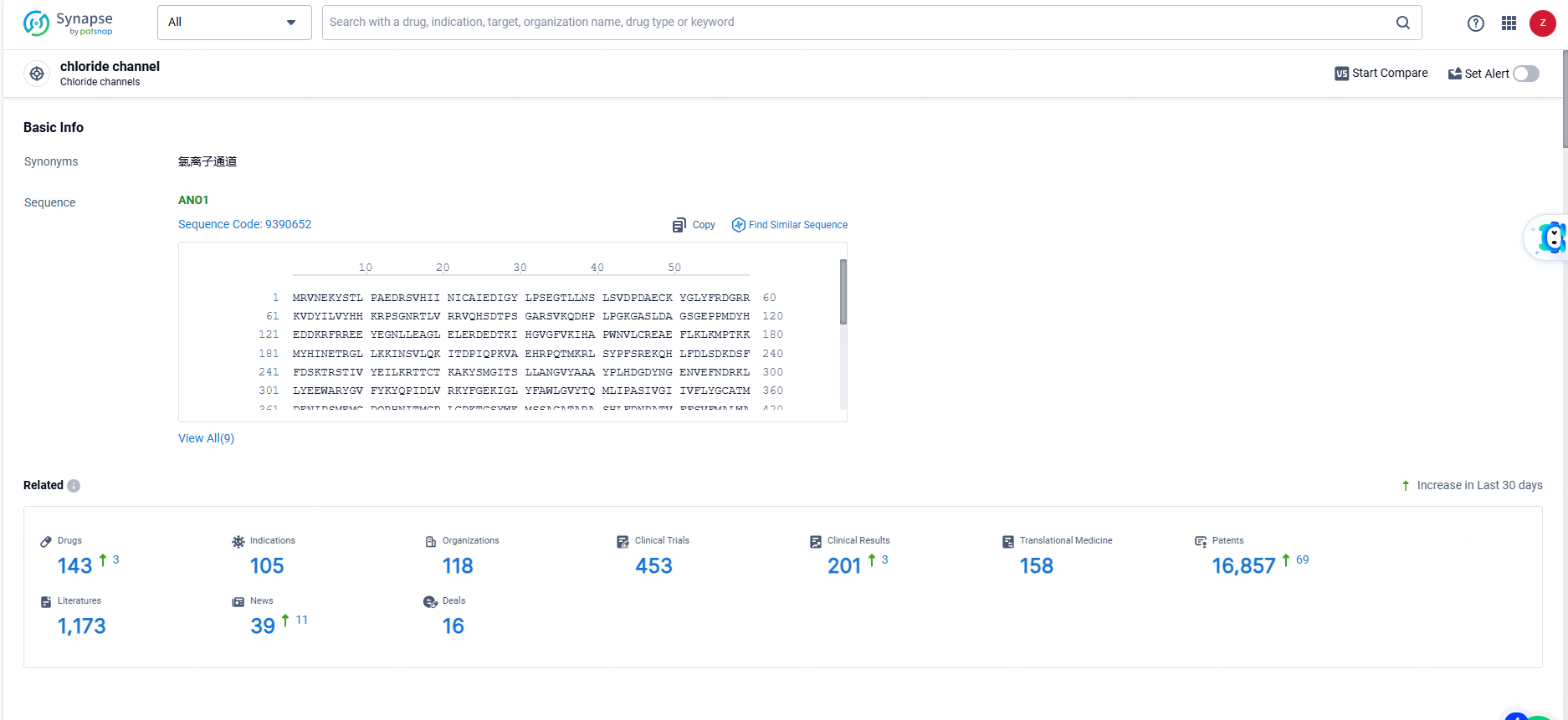
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of June 21, 2024, there are 143 investigational drugs for the chloride channels targets, including 105 indications, 118 R&D institutions involved, with related clinical trials reaching 453, and as many as 16857 patents.
NMD-670 targets chloride channels and currently in Phase 2 of clinical development. With active indications for Myasthenia Gravis and Juvenile Spinal Muscular Atrophy, as well as a designation as an Orphan Drug, NMD-670 shows promise as a potential treatment for a range of diseases across different therap.