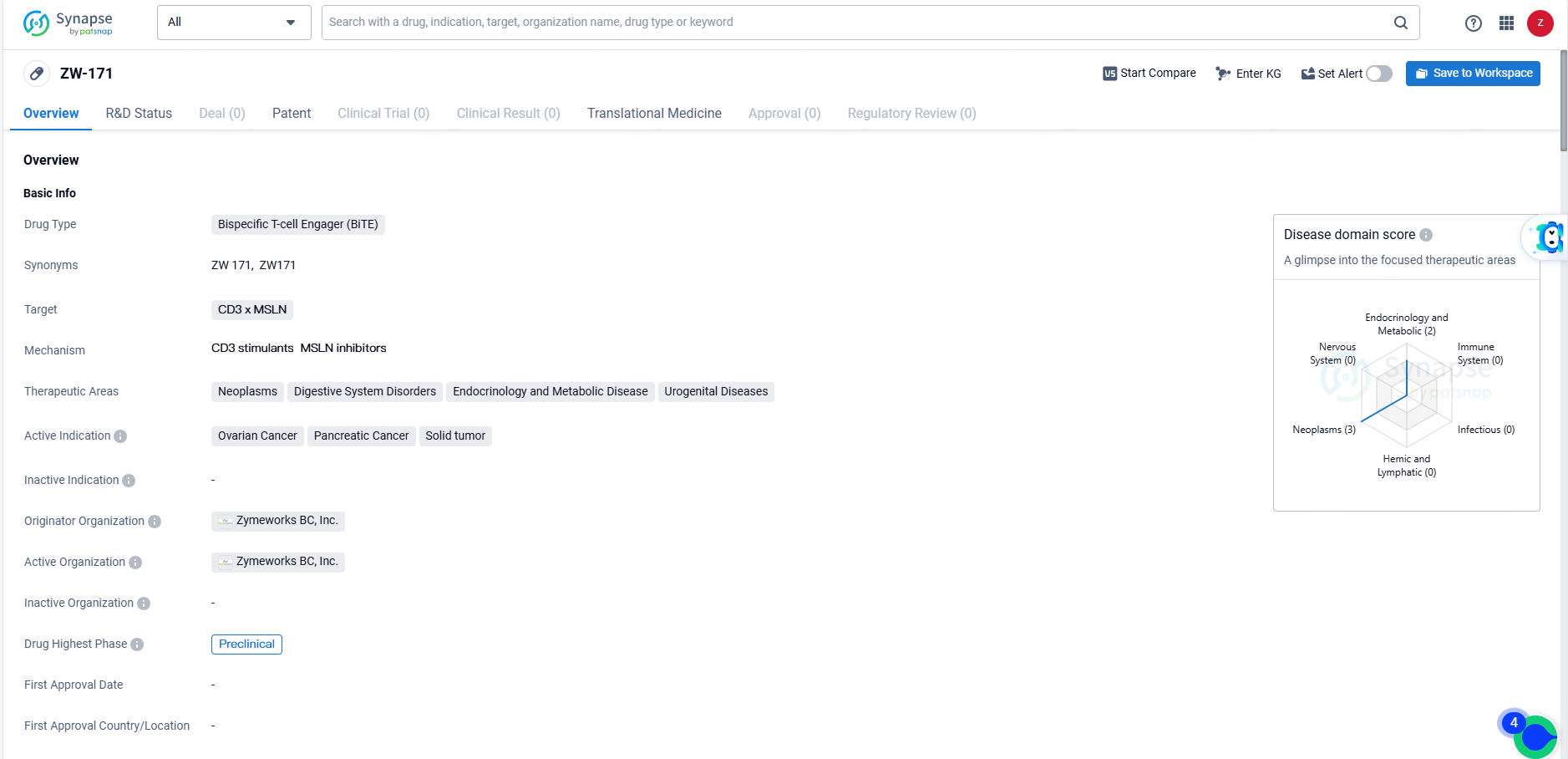
FDA Approves Zymeworks' ZW171, a New Bispecific Antibody for Mesothelin-related Tumors
Zymeworks Inc., a biotechnology company in the clinical development phase, focused on creating a varied array of novel, multifunctional biotherapeutics to enhance treatment standards for challenging diseases, has declared that the United States Food and Drug Administration has approved the investigational new drug application for ZW171. This innovative 2+1 T-cell targeting bispecific antibody is aimed at mesothelin (MSLN)-expressing cancers.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
"We are thrilled to achieve this significant R&D milestone with ZW171, showcasing our dedication to pioneering innovative cancer treatments," commented Paul Moore, the Chief Scientific Officer of Zymeworks. "The preclinical data is encouraging, suggesting that ZW171 could be a more efficient and tolerable therapy for patients suffering from MSLN-expressing malignancies, such as ovarian cancer, non-small cell lung cancer, mesothelioma, and other related cancers. We are enthusiastic about starting the clinical development of ZW171 in 2024 and advancing more candidates in line with our ‘5 by 5’ strategy over the forthcoming 24 months.”
ZW171 is a bispecific antibody engineered to promote T cell-mediated destruction of tumor cells by binding to the extracellular domain of the MSLN protein on tumor cells and engaging CD3 on T cells. Ovarian cancer, non-small cell lung cancer, mesothelioma, and other cancers frequently exhibit moderate to high levels of membranous MSLN expression.
Early evidence of anti-tumor activity with modified T-cell therapy underlines the potential of T-cell targeted approaches in treating MSLN-expressing solid tumors. The unique 2+1 format of ZW171, along with a novel low-affinity anti-CD3 binder, is intended to enhance the therapeutic window by reducing on-target, off-tumor effects and the incidence of cytokine release syndrome while maintaining strong anti-tumor efficacy against MSLN-expressing cancers.
ZW171 is designed to enhance tolerability and anti-tumor activity against MSLN-expressing cancers by selectively targeting tumor cells while sparing normal tissues. Developed and optimized using our proprietary Azymetric™ and EFECT™4 platforms, ZW171 demonstrates superior anti-tumor activity and safety in preclinical models. It effectively induces powerful and selective killing of MSLN-overexpressing cells, while minimizing the risk of on-target, off-tumor effects, peripheral T cell activation, and CRS."
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
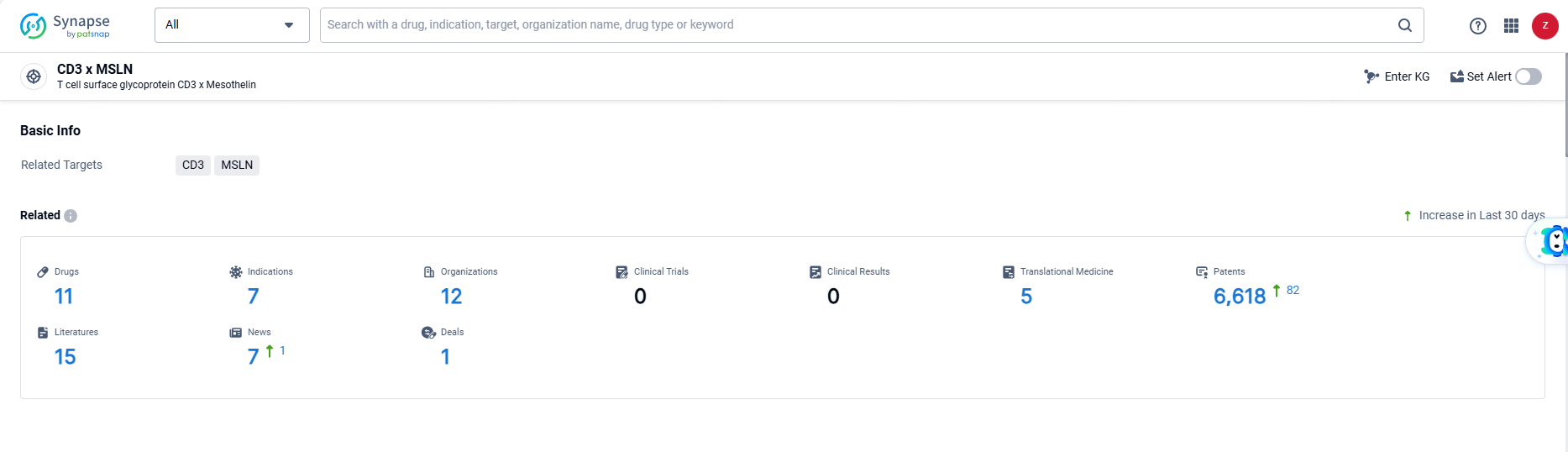
According to the data provided by the Synapse Database, As of June 21, 2024, there are 11 investigational drugs for the CD3 and MSLN targets, including 7 indications, 12 R&D institutions involved, and as many as 6618 patents.
ZW-171 is a bispecific T-cell engager drug targeting CD3 x MSLN, with potential applications in the treatment of various cancers and other related diseases. Its current focus on ovarian cancer, pancreatic cancer, and solid tumors, along with its preclinical development stage, indicates its potential as a novel therapeutic option for patients with these conditions.