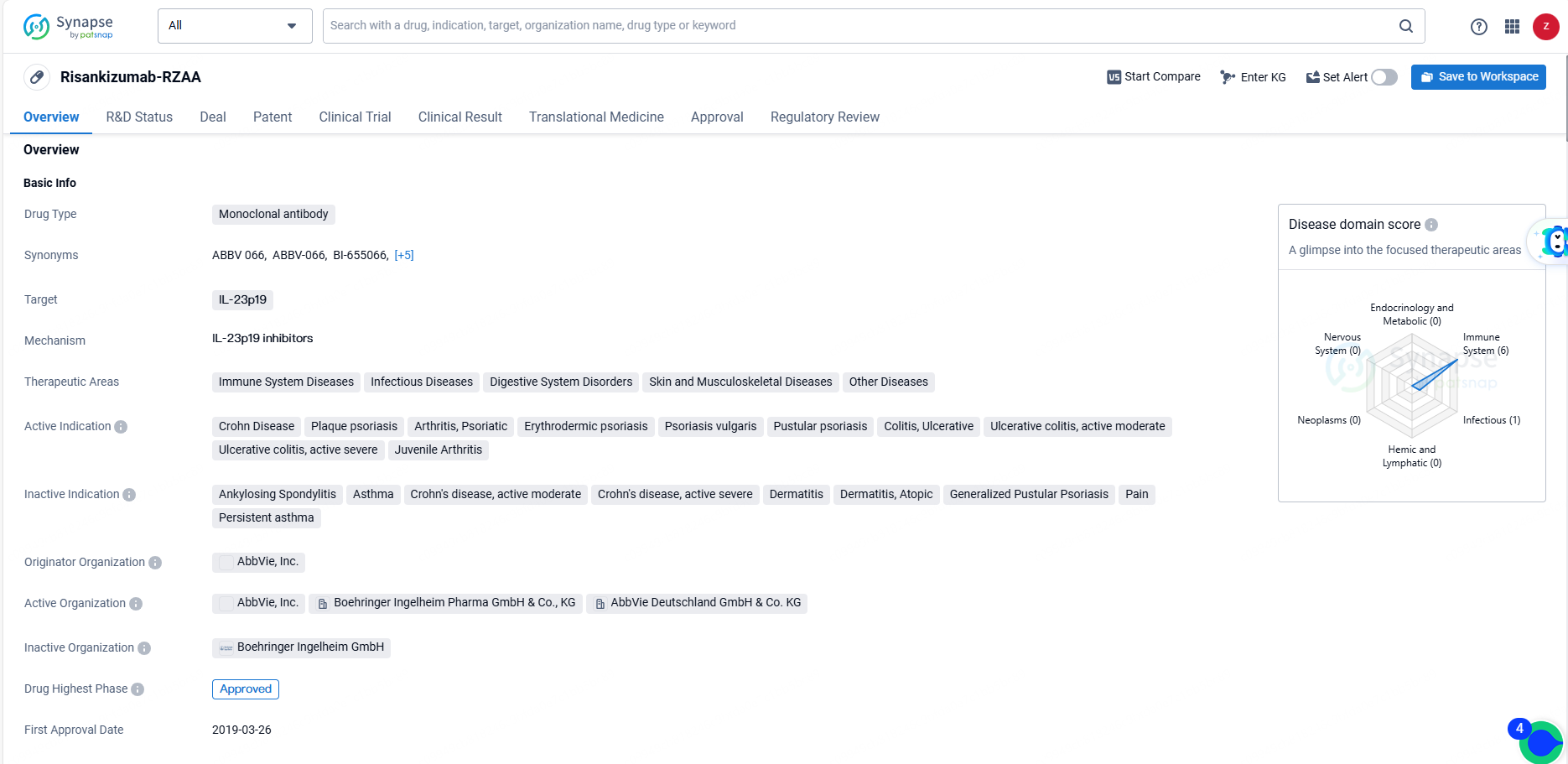
FDA Approves SKYRIZI® for Ulcerative Colitis, Expands AbbVie's Treatment Range
AbbVie revealed that the U.S. Food and Drug Administration has granted approval for SKYRIZI (risankizumab-rzaa) to be used in adults suffering from moderate to severe active ulcerative colitis. This approval marks SKYRIZI as the first IL-23 specific inhibitor to be authorized for both moderate to severe ulcerative colitis and moderate to severe Crohn’s disease. The medication is now sanctioned for four different indications within the scope of immune-mediated inflammatory diseases.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
“When managing patients with ulcerative colitis, it is crucial to focus on both achieving early and sustained clinical remission, along with showing endoscopic improvement,” noted Edward V. Loftus, Jr., M.D., Maxine and Jack Zarrow Family Professor of Gastroenterology at Mayo Clinic in Rochester, Minnesota. “The approval of SKYRIZI represents a significant advancement in reaching these treatment objectives.”
With over a million individuals affected, the United States has one of the highest numbers of people living with ulcerative colitis, and this number is increasing. Ulcerative colitis is a type of inflammatory bowel disease marked by inflammation of the digestive tract, which can result in damage to the colon's lining.
Patients often endure a variety of unpredictable symptoms, such as abdominal pain, bloody stool, and an urgent need to use the restroom, significantly affecting their daily lives. The progression of ulcerative colitis varies among individuals and can, in some cases, lead to surgery or other complications, including cancer or death.
“The authorization of SKYRIZI for ulcerative colitis broadens our IBD treatment options and underscores our dedication to addressing patients' ongoing needs,” said Roopal Thakkar, M.D., senior vice president, chief medical officer, global therapeutics at AbbVie. “We remain committed to investing in innovations that transform the treatment landscape and improve the lives of people afflicted with IBD.”
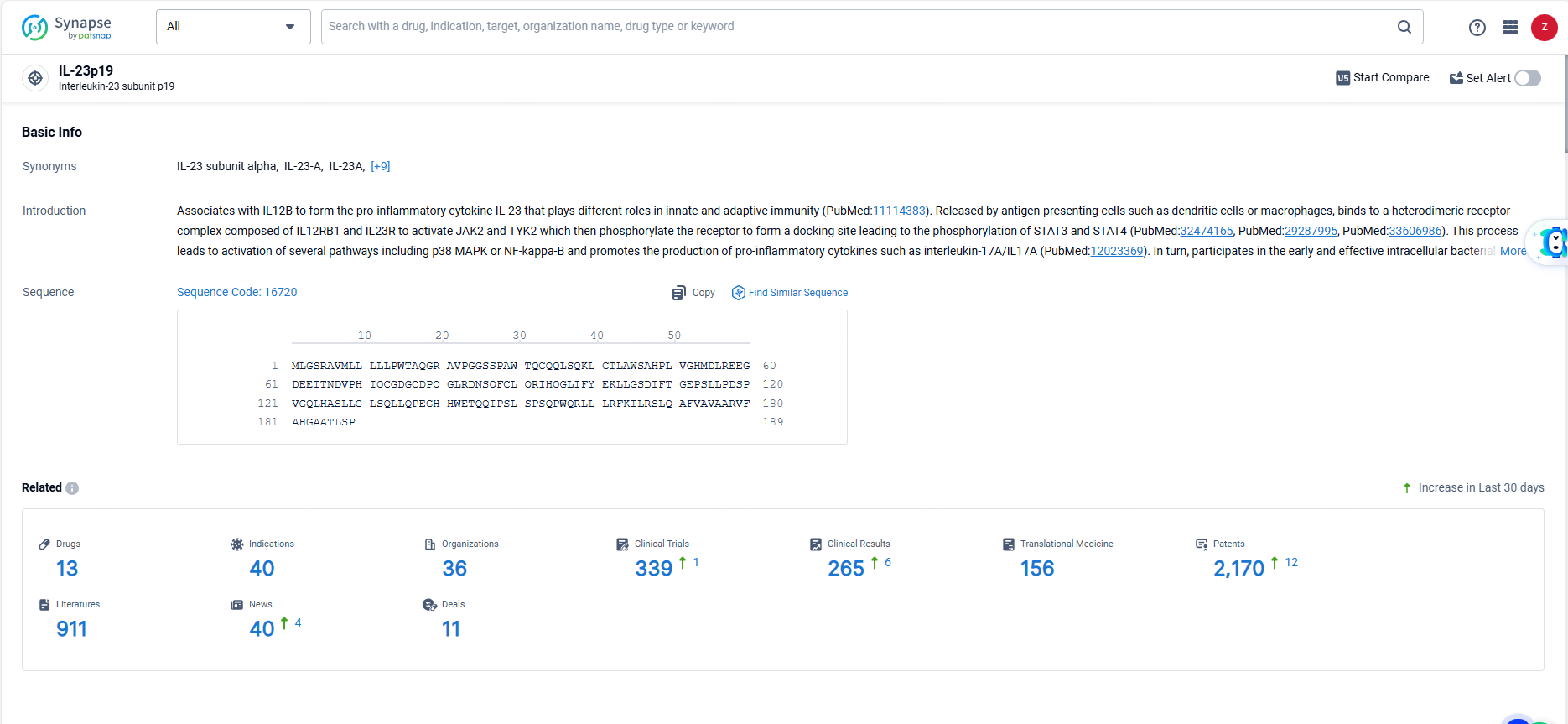
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of June 23, 2024, there are 13 investigational drugs for the IL-23p19 targets, including 40 indications, 36 R&D institutions involved, with related clinical trials reaching 339, and as many as 2170 patents.
Risankizumab-RZAA represents a significant advancement in the treatment of a wide range of diseases, particularly those related to the immune system and inflammatory processes. Its approval in Japan and NDA/BLA status in China indicate its potential to provide therapeutic benefits to patients in these regions. As an orphan drug, it also addresses the unmet medical needs of individuals with rare diseases, further highlighting its importance in the pharmaceutical industry.