The European Commission has approved Sobi®'s ALTUVOCT™ for treating haemophilia A
Sobi revealed that the European Commission has approved the Marketing Authorisation for ALTUVOCT™ (efanesoctocog alfa) for the treatment and prevention of bleeding episodes and perioperative prophylaxis in patients with haemophilia A. ALTUVOCT is a long-lasting factor VIII replacement therapy designed for individuals of all ages and all levels of disease severity.
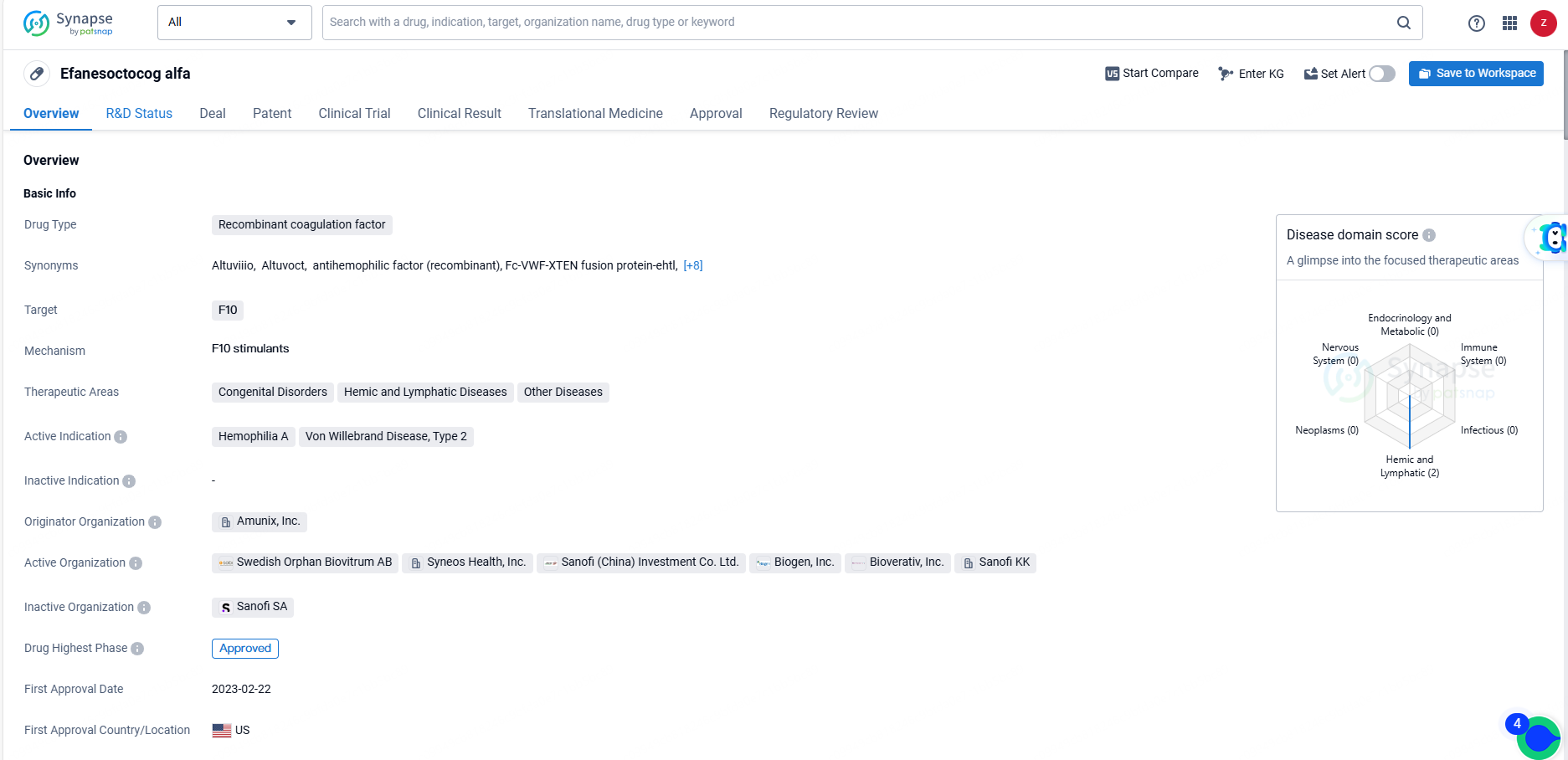
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Children, adolescents, and adults can maintain non-haemophilia factor VIII activity levels for a substantial portion of the week with once-weekly prophylaxis, achieving trough levels of 15% in adults and adolescents prior to the subsequent dose. This provides notably enhanced protection against bleeding compared to previous factor VIII prophylaxis methods.
The European Commission has also approved the European Medicines Agency's (EMA) recommendation to sustain ALTUVOCT’s orphan drug designation, allowing for a 10-year period of market exclusivity. The EMA noted that despite the presence of existing treatments, once-weekly ALTUVOCT prophylaxis significantly reduces the annual bleeding rate compared to other factor VIII therapies, offering a clinically meaningful benefit.
“This announcement is a significant milestone in haemophilia treatment, with the potential to greatly enhance therapeutic outcomes and improve quality of life. For the first time, factor VIII activity levels can be maintained for a considerable part of the week through simplified once-weekly dosing. We are honored to collaborate with the haemophilia community as we spearhead this shift towards normal haemostasis and explore new possibilities,” stated Lydia Abad-Franch, MBA, Head of Research, Development, and Medical Affairs, and Chief Medical Officer at Sobi.
Haemophilia A is a rare, lifelong genetic disorder where the body underproduces or produces dysfunctional factor VIII, a crucial protein for blood clotting. It affects approximately one in 5,000 male births annually and is less common in females. Those with haemophilia may suffer from bleeding episodes that can lead to pain, irreversible joint damage, and life-threatening haemorrhages. Medical outcomes have improved with advancements in available treatments, though there remain significant unmet clinical and social needs for individuals living with this condition.
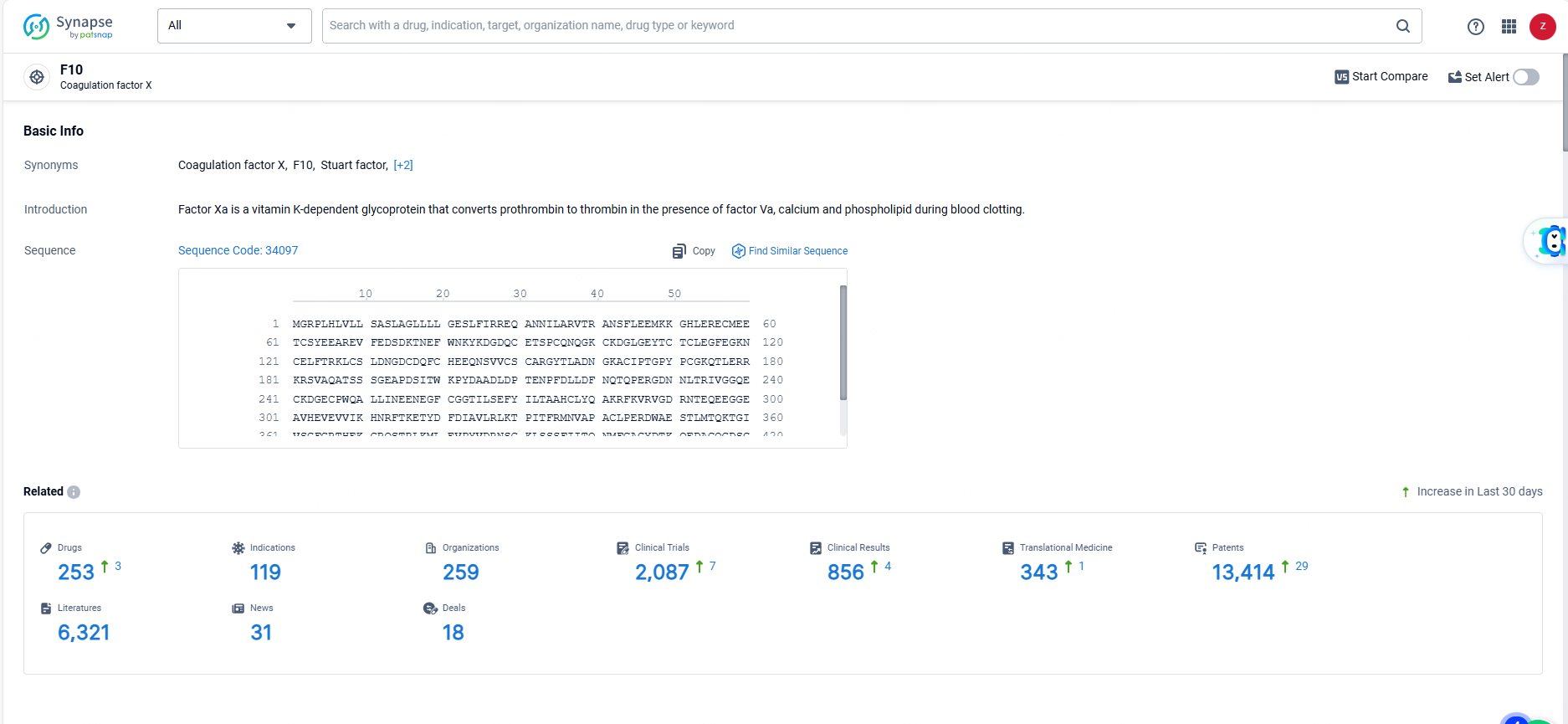
ALTUVOCT was first authorized in the US by the Food and Drug Administration (FDA) in February 2023. Previously, the FDA awarded efanesoctocog alfa Breakthrough Therapy designation in May 2022, marking the first factor VIII therapy to earn this designation, along with Fast Track designation in February 2021, and Orphan Drug designation in 2017.👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of June 23, 2024, there are 253 investigational drugs for the F10 target, including 119 indications, 259 R&D institutions involved, with related clinical trials reaching 2087, and as many as 13414 patents.
Efanesoctocog alfa is a recombinant coagulation factor drug that targets F10 and is used in the treatment of various therapeutic areas including congenital disorders, hemic and lymphatic diseases, and other diseases. As the drug continues to progress through clinical trials and regulatory processes, it is poised to make a meaningful impact in the field of biomedicine and the treatment of coagulation disorders.