FDA Approves Takeda's ENTYVIO® for Crohn’s Disease Treatment
Takeda has declared that the U.S. Food and Drug Administration approved the subcutaneous version of ENTYVIO® (vedolizumab) for use as a maintenance treatment in adult patients experiencing moderate to severe Crohn’s disease, following initial treatment with ENTYVIO given intravenously.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
In September 2023, the FDA granted approval for the subcutaneous (SC) version of ENTYVIO, designed for the maintenance management of adults experiencing moderate to severe ulcerative colitis. This formulation is offered in the United States as a single-dose, prefilled pen.
This approval was supported by the findings of the VISIBLE 2 Study, which was a Phase 3, placebo-controlled, double-blind, randomized trial that evaluated the efficacy and safety of the SC formulation of ENTYVIO for ongoing treatment in adult patients with moderate to severe Crohn's disease (CD). These patients showed a clinical response at Week 6 following initial open-label vedolizumab intravenous administrations at Weeks 0 and 2. The main outcome of the trial was achieving clinical remission at Week 52, anchored on a total Crohn's Disease Activity Index score of ≤150.
"Crohn's disease requires a thoughtful and robust approach to management to prevent its progression. Helping patients reach remission is a fundamental objective of mine," stated Timothy Ritter, MD, GI Alliance Research’s senior medical director, Department of Research and Education, and assistant professor at TCU School of Medicine. "The results from VISIBLE 2 underscore ENTYVIO’s solid efficacy, which holds irrespective of administration method, with nearly 50% of participants on ENTYVIO SC maintaining long-term remission."
During the VISIBLE 2 trial, 409 participants were blindly randomized in a 2:1 ratio at Week 6 to receive either ENTYVIO 108 mg through SC injections or a placebo biweekly. This included patients who had not responded adequately to or could not tolerate one or more treatments, such as corticosteroids, immunomodulators, or TNF blockers.
"The introduction of subcutaneous ENTYVIO for Crohn's disease aligns with our commitment to enhancing therapeutic strategies for ulcerative colitis and Crohn's disease, ensuring patient-centric care with multiple administration options," commented Brandon Monk, Takeda’s senior vice president and head of the U.S. Gastroenterology Business Unit. "With the ENTYVIO Pen, patients gain the convenience of self-administering their maintenance therapy, whether at home or while traveling.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
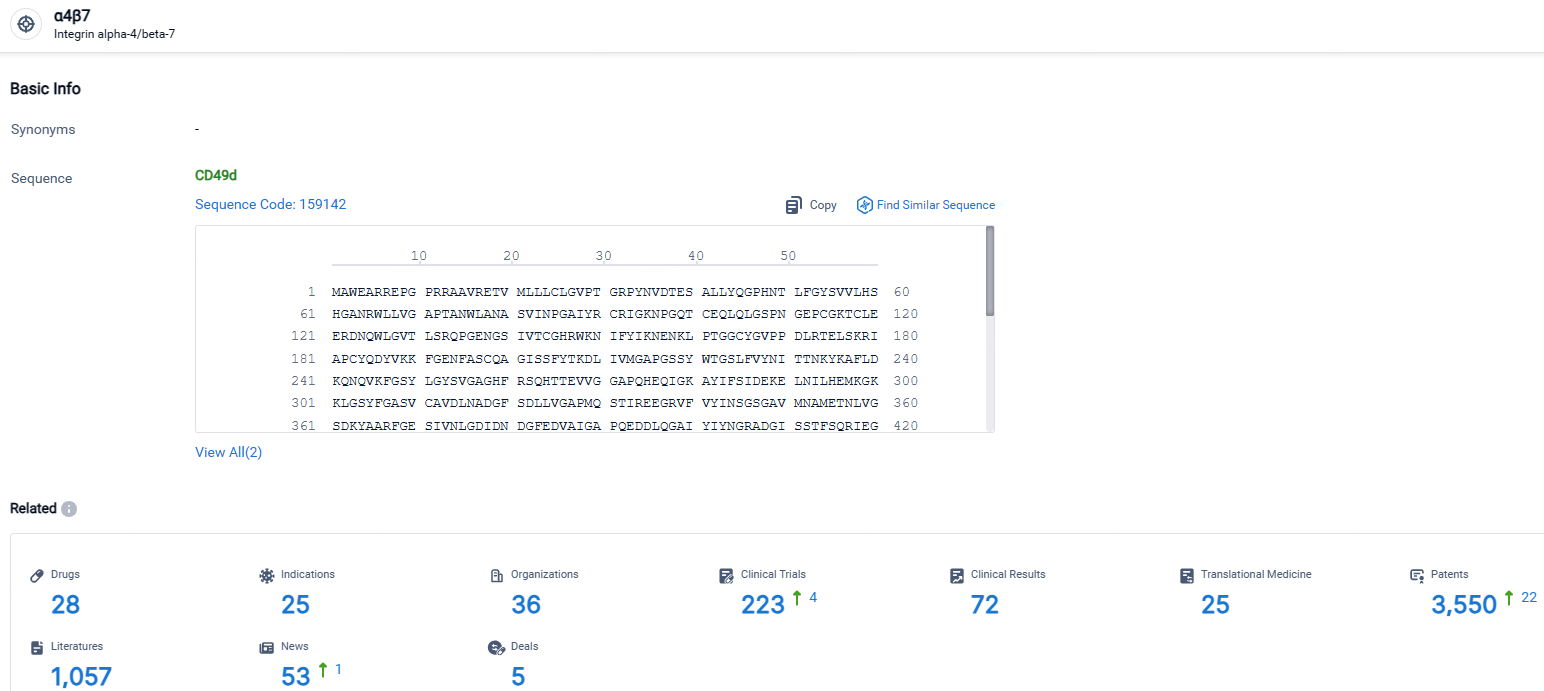
According to the data provided by the Synapse Database, As of April 23, 2024, there are 28 investigational drugs for the α4β7 receptor, including 25 indications, 36 R&D institutions involved, with related clinical trials reaching 223, and as many as 3550 patents.
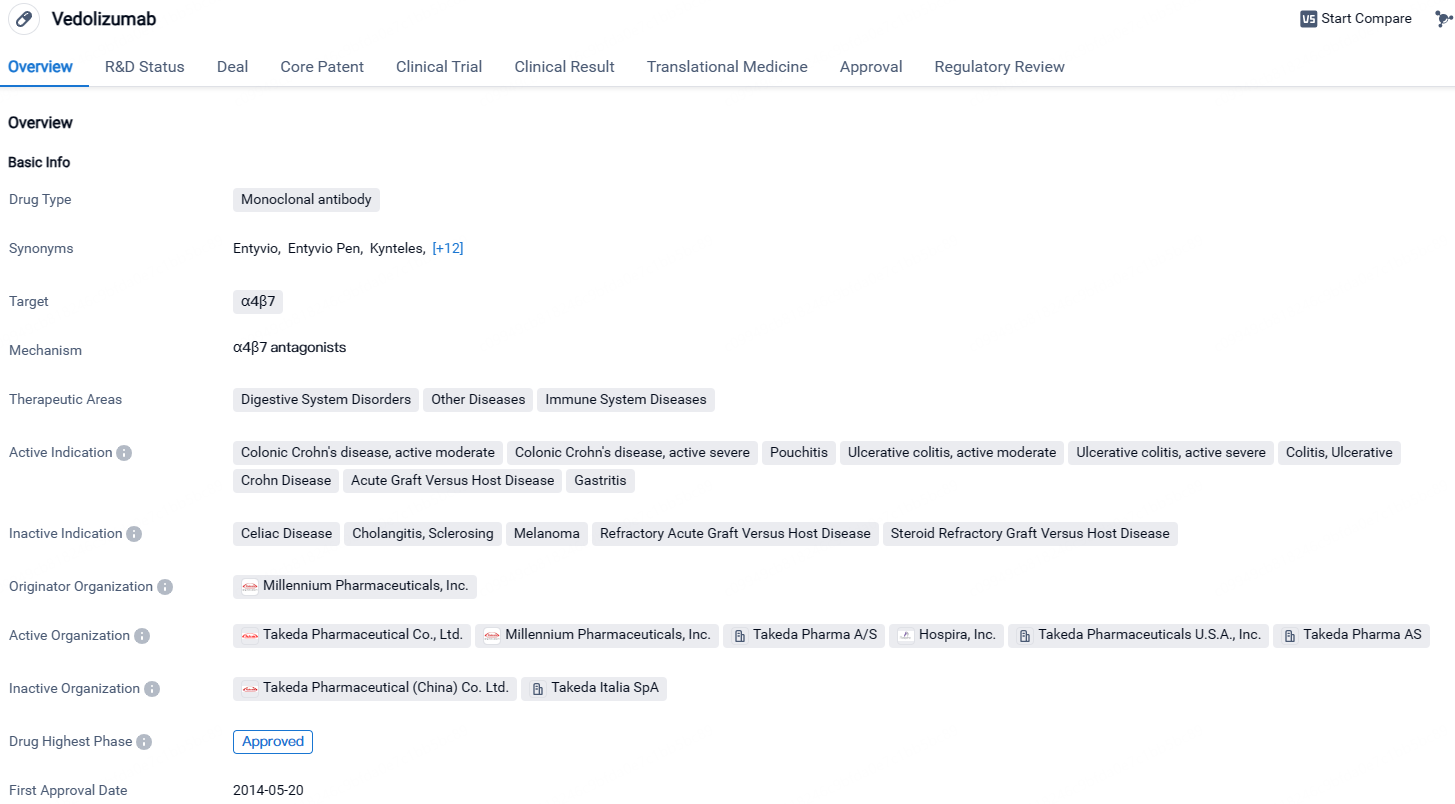
Vedolizumab is a monoclonal antibody drug that targets α4β7 and is approved for the treatment of various digestive system disorders, immune system diseases, and other conditions. Its approval in both the United States and China highlights its potential therapeutic benefits. The drug's regulatory designations further emphasize its importance in addressing unmet medical needs and treating serious conditions.