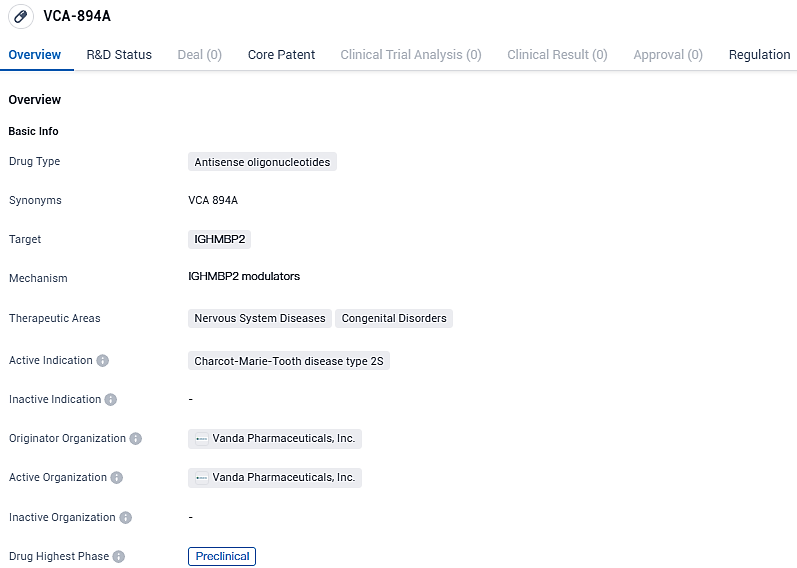
FDA Approves Vanda Pharmaceuticals' VCA-894A Therapy for Charcot-Marie-Tooth Disease 2S
Vanda Pharmaceuticals Inc. has made a public statement confirming the sanctioning by the U.S. Food and Drug Administration of their Investigational New Drug submission. This allows for the clinical assessment of VCA-894A as a potential therapeutic agent for an individual diagnosed with Charcot-Marie-Tooth disease, specifically the axonal type 2S, which arises due to hidden splice site mutations present in the IGHMBP2 gene.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
CMT2S is an uncommon form of Charcot-Marie-Tooth disease, a genetic disorder affecting the peripheral nerves, for which effective treatments are currently unavailable. This particular subtype is marked by a gradual decrease in muscular strength and wasting, predominantly in the extremities, starting in the first ten years of a patient's life. Those afflicted exhibit diminished reflexes and a loss of sensory functions.
Charcot-Marie-Tooth disease has an estimated occurrence of 1 per 2,500 people. The clinical manifestations vary due to the different genetic mutations linked to the disease. The CMT2S mutation, in particular, has an estimated global incidence of fewer than 1 per million individuals.
Mihael H. Polymeropoulos, M.D., the President, CEO, and Chairman of Vanda Pharmaceuticals, expressed that this development signifies a significant step towards achieving precise medical solutions that can craft treatments based on individual genetic profiles, specifically targeting mutations responsible for CMT2S.
The experimental compound VCA-894A is an innovative antisense oligonucleotide designed to directly target and modify the activity of a hidden splicing site in the IGHMBP2 gene. This gene's alterations are critical in the development of CMT2S, often leading to the loss of alpha-motor neurons and thus prompting the decline of the peripheral nervous system.
Antisense oligonucleotides have the ability to influence gene behavior, offering an avenue for individualized approaches to treating uncommon illnesses. The administration of antisense oligonucleotides into the central nervous system has already proven effective in various programs, presenting a versatile solution for many neurodegenerative and neuromuscular conditions.
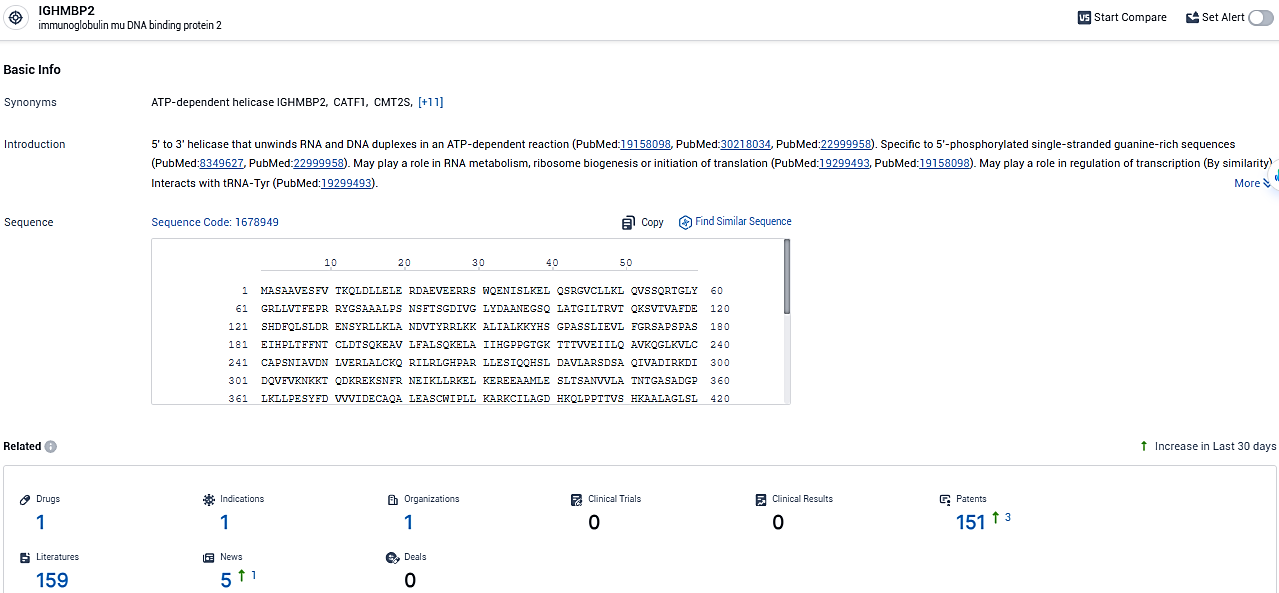
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of January 29, 2024, there are 1 investigational drugs for the IGHMBP2 target, including 1 indications, 1 R&D institutions involved, and as many as 151 patents.
VCA-894A specifically targets a cryptic splice site variant within IGHMBP2, which causes CMT2S. ASOs may have broad applicability in addressing a number of disorders, from nervous system treatments to systemic treatments. Currently in the preclinical phase, VCA-894A holds orphan drug status, highlighting its potential to address an unmet medical need in this specific patient population.