FDA Clears IND for Umoja's UB-VV111: A CD19 CAR T-Cell Therapy for Blood Cancers
Umoja Biopharma, Inc. has received approval for its Investigational New Drug application from the U.S. Food and Drug Administration concerning UB-VV111, a gene therapy that produces CD19 CAR T-cells directly within the patient's body, designed to address hematologic cancers. Umoja plans to start a Phase 1 clinical trial and administer the first dose to a patient before the close of 2024. UB-VV111 represents the inaugural application from the VivoVecTM gene delivery platform to reach clinical trials.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
“The IND clearance for UB-VV111 marks a pivotal achievement in Umoja’s commitment to developing readily available therapies that address the shortcomings of existing ex vivo cellular immunotherapies,” stated Andrew Scharenberg, M.D., Co-Founder and CEO of Umoja. “We are thrilled to be at the forefront of the in vivo field, working to eliminate the numerous obstacles and challenges associated with early-generation ex vivo CAR T-cell therapies, including the complex, lengthy, and expensive production process and the intricate administration procedure. Clinicians and patients have long awaited an improved solution, and we are eager to commence our inaugural clinical trial.”
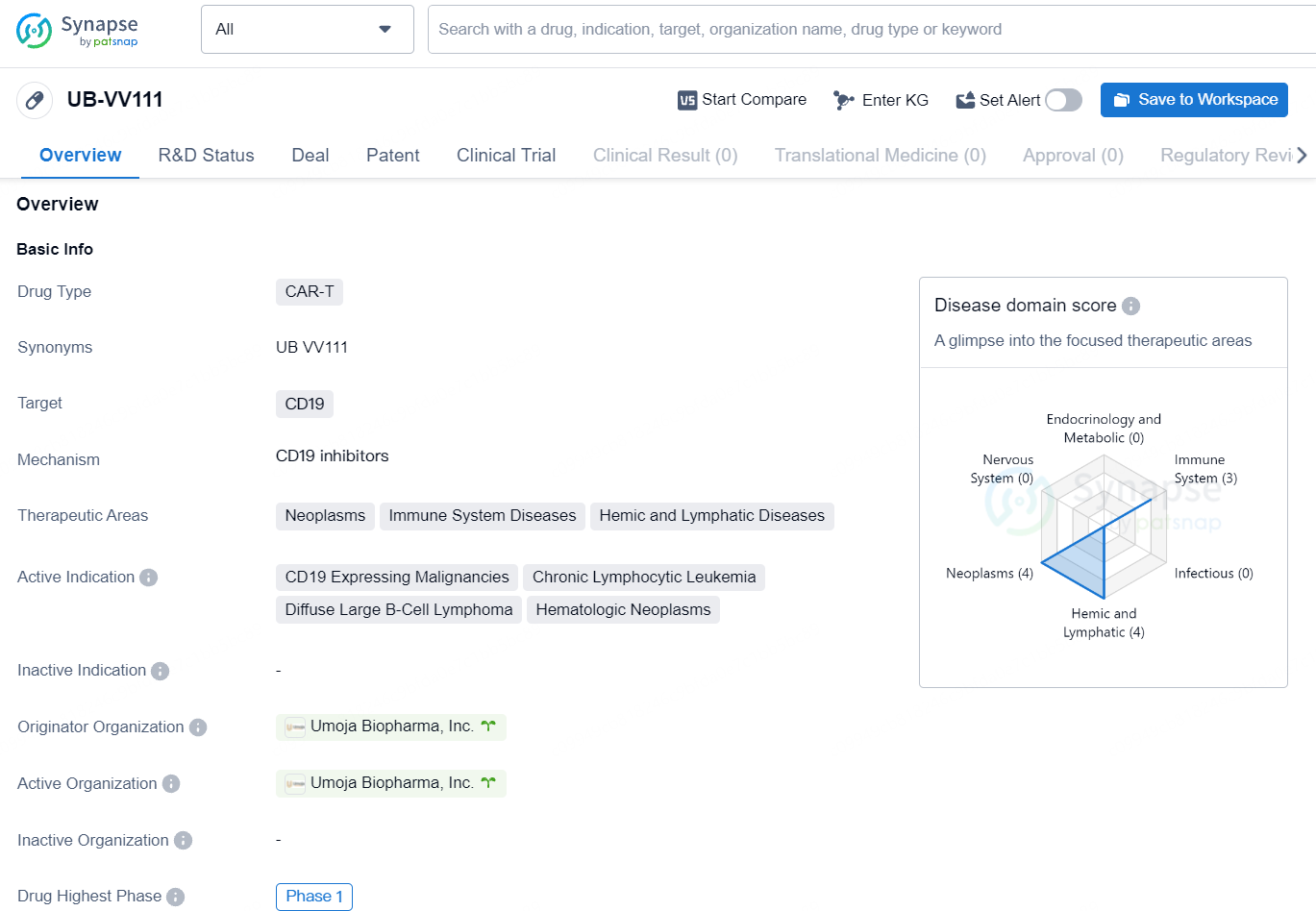
The Phase 1 trial for UB-VV111 will involve a dose escalation and confirmation study designed to assess the safety, tolerability, and clinical antitumor efficacy of UB-VV111. The study will enroll participants with relapsed/refractory large B-cell lymphoma and chronic lymphocytic leukemia, covering both CAR T naïve and CAR T treated individuals.
In January 2024, Umoja Biopharma and AbbVie disclosed two exclusive option and license agreements aimed at developing several in situ generated CAR T-cell therapy candidates. These candidates are targeted primarily at oncology, with potential uses in immunology. As part of the agreements, AbbVie holds an exclusive option to license Umoja’s CD19-directed in situ generated CAR T-cell therapy candidates, including UB-VV111.
UB-VV111 is a lentiviral vector-based gene therapy stemming from Umoja’s VivoVec platform, featuring a surface-engineered viral envelope. UB-VV111 includes a transgene for an anti-CD19 CAR and a Rapamycin Activated Cytokine Receptor, intended to enrich and expand UB-VV111 engineered CAR T cells in vivo. UB-VV111 is being tested for various B-cell malignancies, such as Large B-Cell Lymphoma and Chronic Lymphocytic Leukemia.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
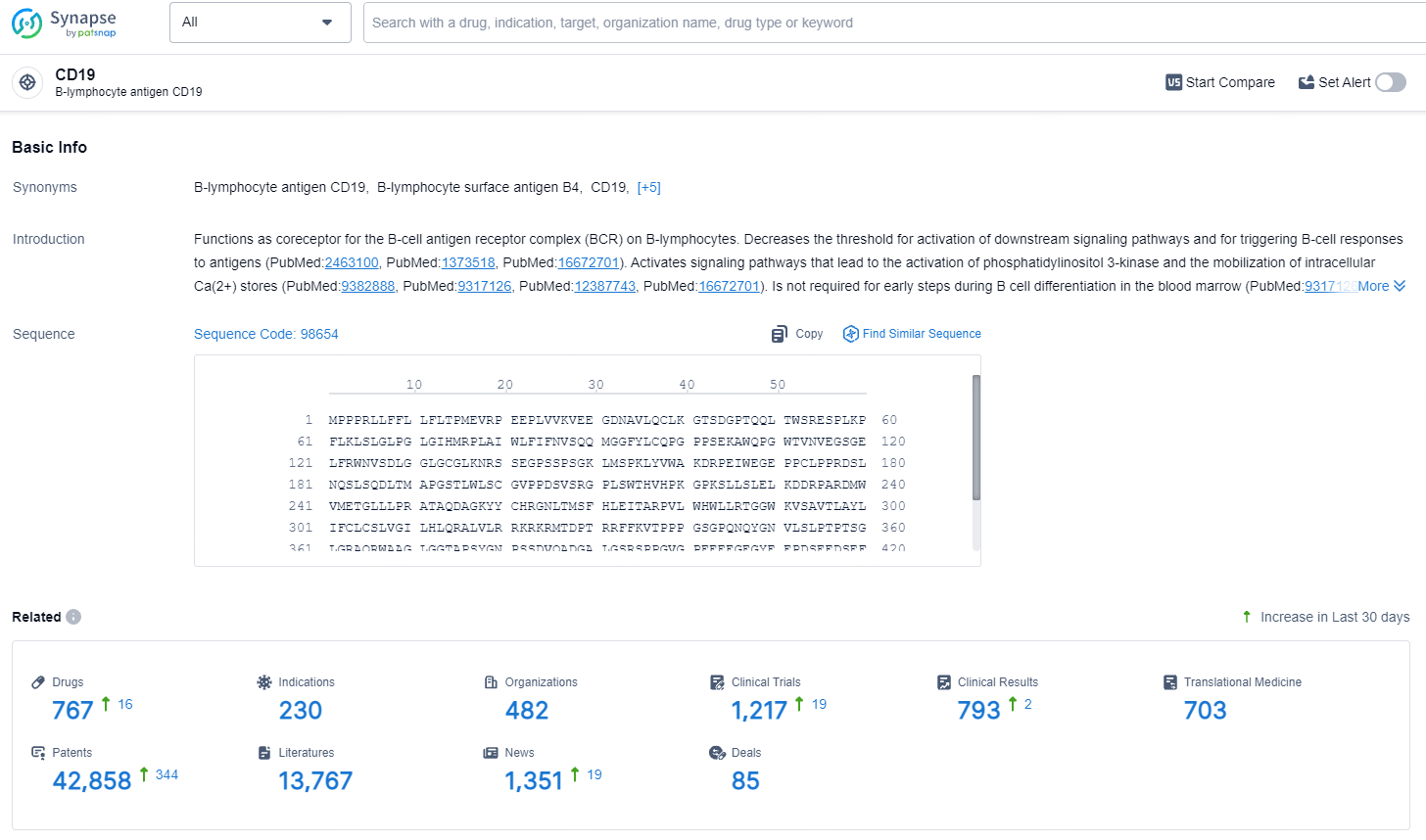
According to the data provided by the Synapse Database, As of August 5, 2024, there are 767 investigational drugs for the CD19 targets, including 230 indications, 482 R&D institutions involved, with related clinical trials reaching 1217, and as many as 42858 patents.
UB-VV111 is a CAR-T therapy targeting CD19 with a focus on neoplasms, immune system diseases, and hemic and lymphatic diseases. Its active indication includes CD19 expressing malignancies, chronic lymphocytic leukemia, diffuse large B-cell lymphoma, and hematologic neoplasms. UB-VV111 has reached the highest global phase of Phase 1 clinical trials, holding promising potential for the treatment of various malignancies.