Connext Successfully Gives Initial CNT201 Dose for Dupuytren’s Contracture
Connext has revealed the initial successful administration of CNT201, its therapeutic solution for Dupuytren’s contracture, in patients. Dupuytren’s contracture is a severe ailment marked by the fibrotic alteration of the fascia located between the skin and tendons in the palm as a result of collagen accumulation, leading to permanently bent fingers and considerably diminishing the quality of life for those affected. At present, there is no definitive cure available for this condition.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Primarily, treatments for alleviating symptoms often include surgical interventions such as fasciectomy. However, non-surgical approaches, like localized collagenase injections, have yielded high patient satisfaction.
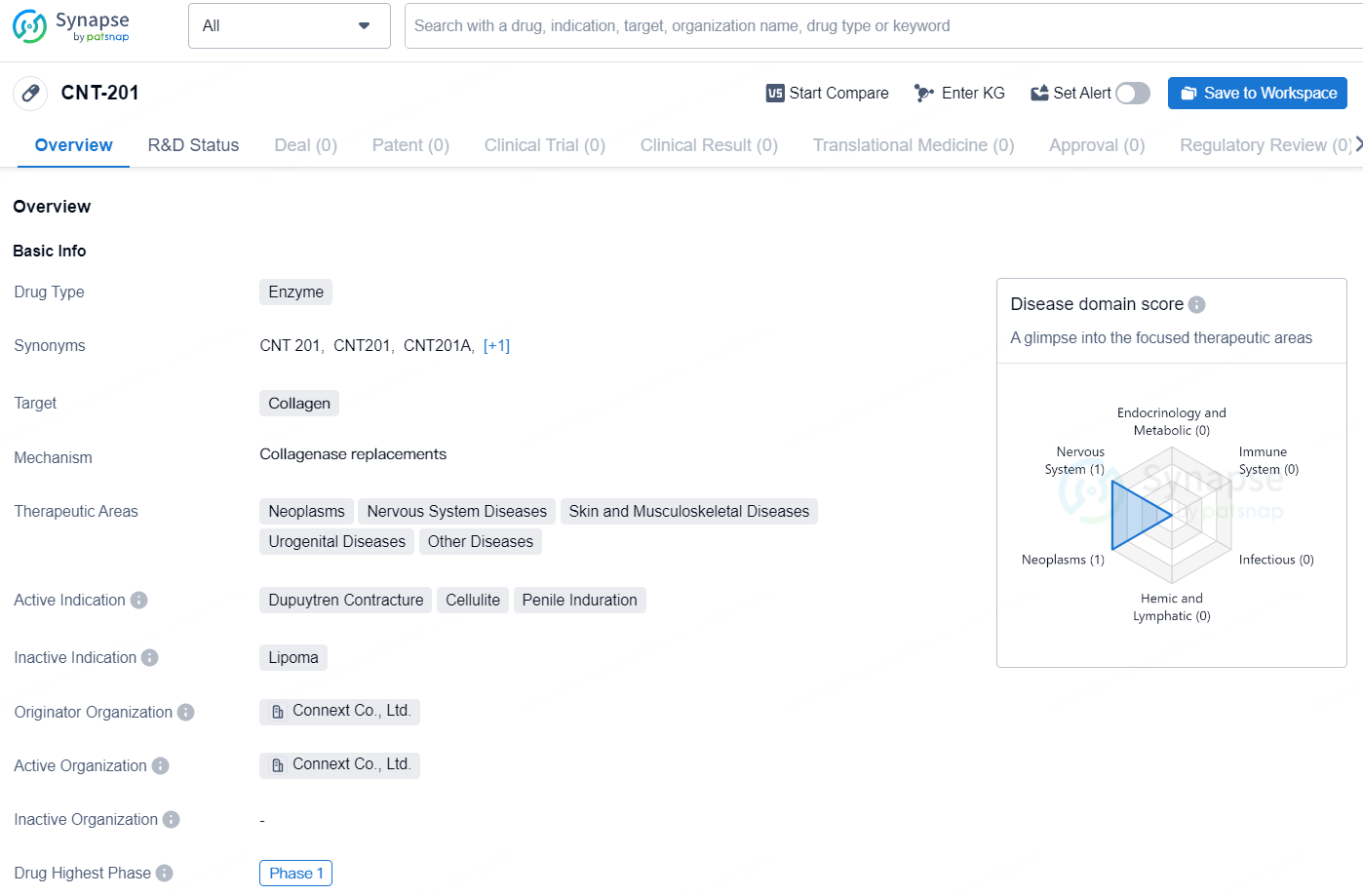
The U.S. FDA has granted IND approval for Phase 1/2 clinical trials, and the Clinical Trial Notification process in Australia has recently concluded. In Australia, the ongoing Phase 1 portion of the Phase 1/2 trial is taking place across three hospitals.
The study entails single administrations of escalating doses of four levels in patients with Dupuytren’s contracture, followed by a four-week evaluation period to gauge safety, tolerability, efficacy, pharmacokinetics, and immunogenicity. Connext aims to strengthen proof-of-concept data by mid-next year and identify the optimal dose for Phase 2, targeting the start of the Phase 2 trial in the latter half of the year.
Connext CEO Woojong Lee stated that the first administration represents a crucial milestone, providing new hope for patients with Dupuytren’s contracture. He emphasized their commitment to developing a treatment that is safer and more effective to enhance patient quality of life.
At the end of last year, Connext successfully completed a Series B investment round and was chosen for the Scale-Up TIPS program, facilitating the launch of global clinical trials for Dupuytren’s contracture. Concurrently, the company is also advancing the development of additional indications, including Peyronie’s disease and cellulite.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
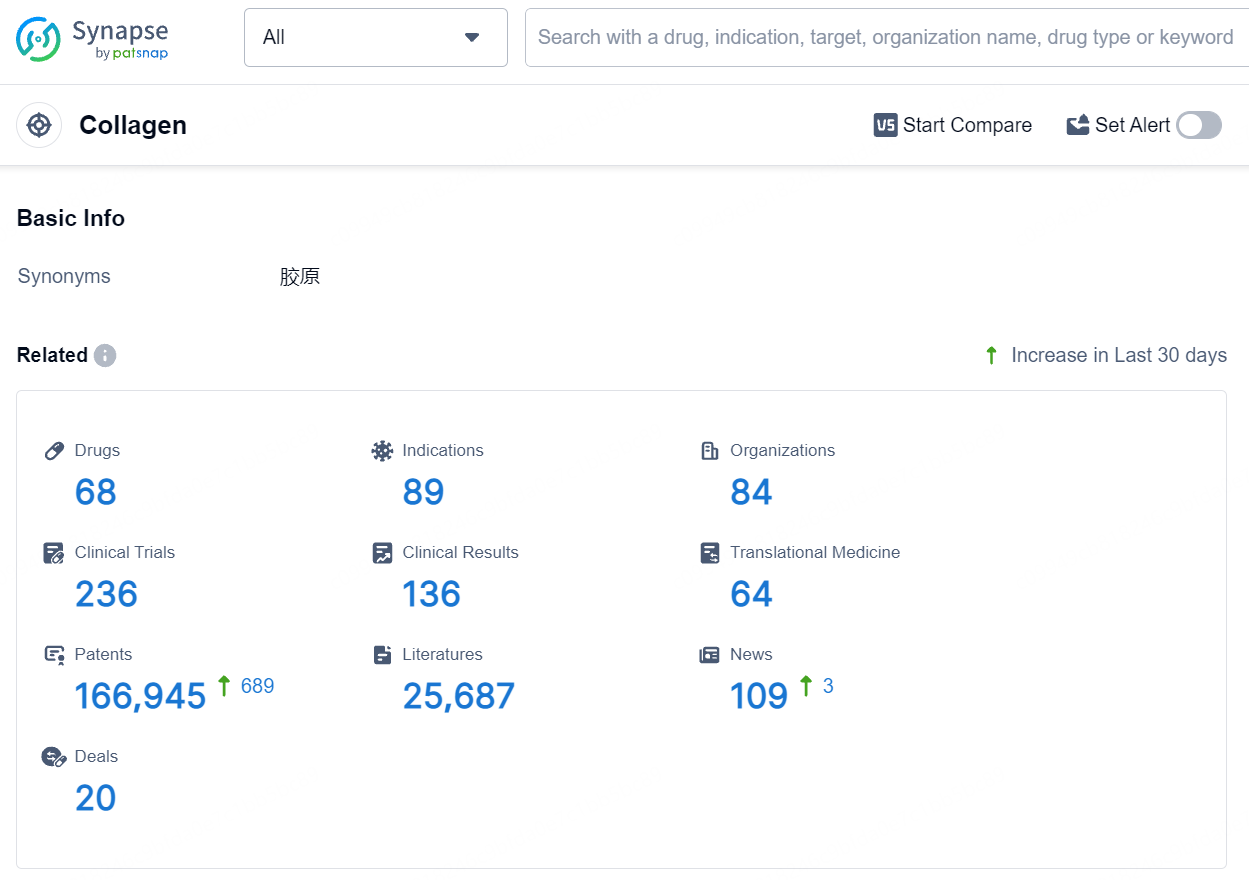
According to the data provided by the Synapse Database, As of August 5, 2024, there are 68 investigational drugs for the collagen target, including 89 indications, 84 R&D institutions involved, with related clinical trials reaching 236, and as many as 166945 patents.
CNT-201 is an enzyme-based drug developed by Connext Co., Ltd. with a focus on targeting collagen for the treatment of a wide range of diseases. The drug has shown potential in its initial clinical development, reaching Phase 1 globally. However, further information on the drug's development and potential market availability would be needed in order to fully assess its potential impact in the pharmaceutical industry.