FDA Fast-Tracks Review of Supplements to TIVDAK® License for Those Suffering from Advanced Cervical Cancer Relapse or Spread
Pfizer Inc. and Genmab A/S have publicized that their supplemental Biologics License Application for TIVDAK (tisotumab vedotin-tftv) has been acknowledged by the U.S. Food and Drug Administration. This submission is aimed at transforming TIVDAK's existing accelerated approval into a full approval status. Intended for patients who have recurrent or metastatic cervical cancer and have witnessed disease advancement following or during their initial treatment, TIVDAK's review has been given an expedited status under the Priority Review designation. In line with the Prescription Drug User Fee Act, a target action date has been set for May 9, 2024.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The results from the third phase of the innovaTV 301 study have shown a positive balance between the advantages and the risks, with increases in overall patient survival, reinforcing the evidence that supports the use of TIVDAK for individuals suffering from recurrent and metastatic cervical cancer, according to Roger Dansey, M.D., the lead figure for Oncology Development at Pfizer. Dansey also notes the critical development of the FDA's review of their submitted sBLA, which is a step forward in providing a life-prolonging option for more adults with cervical cancer.
This sBLA draws its strength from the comprehensive data on both the effectiveness and safety from the international, multicenter, Phase 3 innovaTV 301 trial. The study showcased TIVDAK's superior efficacy in improving overall survival, halting disease progression, and achieving verifiable responses as adjudicated by researchers, in those patients with cervical cancer that had recurred or spread after prior treatment. The safety patterns observed with TIVDAK in innovaTV 301 remained in line with the pre-established safety information disclosed in its U.S. prescribing documentation. Notably, the innovaTV 301 study findings were brought to light at the esteemed Presidential Symposium hosted by the European Society of Medical Oncology Congress in October 2023.
The U.S. documents detailing TIVDAK's use caution users with a prominently placed warning regarding potential eye-related side effects, in addition to advising on risks such as peripheral neuropathy, bleeding complications, lung inflammation, serious skin reactions, and dangers to developing fetuses. Further detailed Safety Information is provided beneath.
“With the ongoing need for new therapeutic interventions for metastatic cervical cancer that offer not just longer survival but also a unique approach to combating the disease, this juncture is critical," remarked Jan van de Winkel, Ph.D., the CEO at Genmab. “This landmark reiterates our dedication to make TIVDAK available to American women diagnosed with cervical cancer who have seen disease progression after initial treatment.”
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
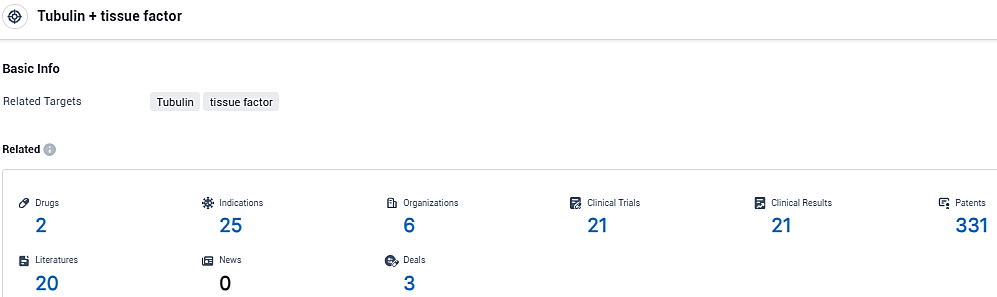
According to the data provided by the Synapse Database, As of January 13, 2024, there are 2 investigational drugs for the Tubulin+ tissue factor tagets, including 25 indications, 6 R&D institutions involved, with related clinical trials reaching 21, and as many as 331 patents.
TIVDAK (tisotumab vedotin-tftv) is an antibody-drug conjugate composed of Genmab’s human monoclonal antibody directed to tissue factor and Pfizer’s ADC technology that utilizes a protease-cleavable linker that covalently attaches the microtubule-disrupting agent monomethyl auristatin E to the antibody. Determination of TF expression is not required. TIVDAK was granted accelerated approval in the U.S. by the FDA in September 2021.