Astellas Issues Progress Report on U.S. Regulatory Submission for Zolbetuximab Biological License Application
Astellas Pharma Inc. disclosed that on January 4, 2024, the U.S. Food and Drug Administration provided a complete response letter pertaining to the submitted Biologics License Application for zolbetuximab. This experimental therapeutic is designed for administration to individuals diagnosed with locally advanced unresectable or metastatic HER2-negative gastric or gastroesophageal junction adenocarcinoma, specifically targeting patients whose tumors express the claudin 18.2 biomarker.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The FDA has communicated that it will not be able to provide a green light for the Biologics License Application (BLA) for zolbetuximab by the deadline stipulated by the Prescription Drug User Fee Act (PDUFA), set for January 12, 2024. This delay arises from certain outstanding issues noted during the FDA's inspection of an external manufacturer involved in the production of zolbetuximab.
No issues have been raised by the FDA concerning zolbetuximab's clinical trial findings on its effectiveness and safety. Furthermore, the FDA does not seek any new clinical trials. Astellas is actively collaborating with both the FDA and the external manufacturer to outline a plan to swiftly address the issues identified by the FDA. Astellas confirms that this situation does not impact any of their other products.
Astellas' leadership expresses their belief in the efficacy and the importance of zolbetuximab for addressing the medical needs of patients with advanced gastric or gastroesophageal junction (GEJ) cancer, especially those expressing the CLDN18.2 biomarker. Dr. Moitreyee Chatterjee-Kishore, Senior Vice President and Leader of Immuno-Oncology Development at Astellas, has affirmed the company's dedication to cooperating with the FDA and the contract manufacturer in resolving the identified issues. The goal is to make zolbetuximab available to patients in the U.S. who require it without unnecessary delay.
In its mechanism, zolbetuximab functions as an antibody that specifically targets and binds to the CLDN18.2 antigen on the surface of cancer cells within the gastric epithelium. Previous laboratory studies have shown that once zolbetuximab attaches to cancer cells, it activates the immune response to attack the cells through two mechanisms: antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity, leading to the destruction of the cancer cells.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
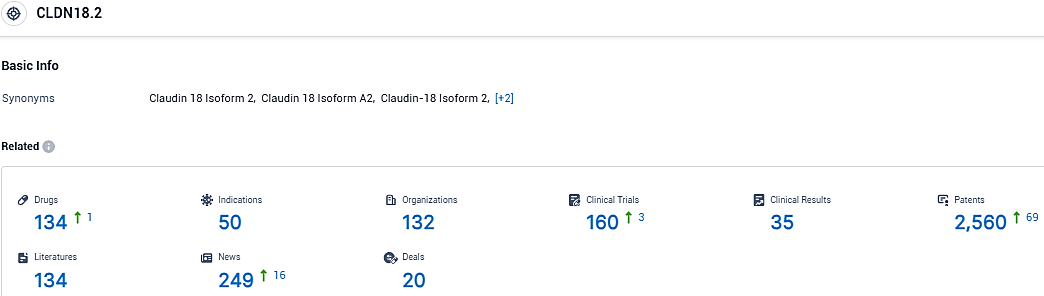
According to the data provided by the Synapse Database, As of January 13, 2024, there are 134 investigational drugs for the CLDN18.2 tagets, including 50 indications, 132 R&D institutions involved, with related clinical trials reaching 160, and as many as 2560 patents.
Zolbetuximab is an investigational, first-in-class chimeric IgG1 monoclonal antibody that targets and binds to claudin 18.2, a transmembrane protein. Zolbetuximab has not been approved by any regulatory bodies for the treatment of patients with gastric and GEJ cancers, and there is no guarantee the agent will receive regulatory approval or become commercially available for the uses being investigated.