FDA Grants Approval for WestGene's mRNA Cancer Treatment Vaccine
WestGene, committed to advancing mRNA technology, proudly declares a significant breakthrough as the FDA grants IND approval for its mRNA-based cancer vaccine, WGc-043. This pivotal success signifies the global debut of an EB virus-associated mRNA therapeutic cancer vaccine's approval.
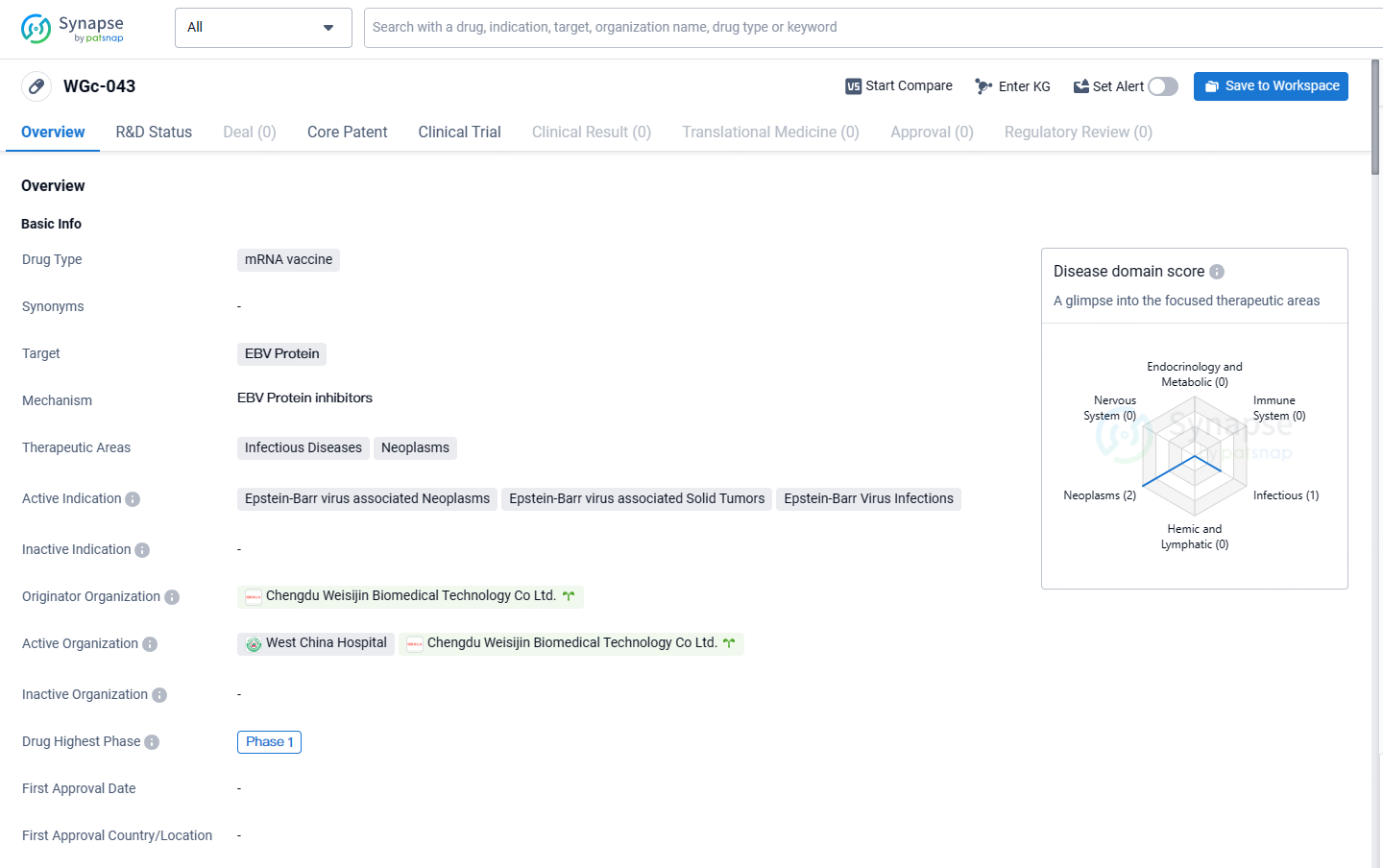
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Established under the guidance of the esteemed Dr. Yuquan Wei, a member of the Chinese Academy of Sciences, as well as Dr. Xiangrong Song, WestGene has emerged as a key player in the field of mRNA technology exploration and pioneering pharmaceutical development. The firm's ongoing dedication to scientific distinction is evident in its recent achievements, symbolizing its dedication to pushing the boundaries of biomedicine.
The U.S. Food and Drug Administration's approval of WGc-043 marks a major breakthrough in the field of oncology, presenting renewed optimism for patients suffering from advanced cancers linked to the EB virus. The EB virus is strongly associated with various types of malignancies such as nasopharyngeal carcinoma, natural killer T-cell lymphoma, and cancers of the stomach, lungs, liver, esophagus, breast, cervix, as well as autoimmune conditions like multiple sclerosis and systemic lupus erythematosus. These conditions represent potential uses for WGc-043.
In trials, WGc-043 has demonstrated promising results in terms of effectiveness, low toxicity, wide-ranging suitability, scalable production, and affordability. Results from investigator-led trials in nasopharyngeal carcinoma and natural killer T-cell lymphoma have shown that WGc-043 offers outstanding safety and effectiveness over other available mRNA-based cancer vaccines. Once introduced to the market, WGc-043 is expected to be a novel therapeutic choice for patients battling advanced EB virus-induced solid and blood cancers.
Looking ahead, WestGene emphasizes strategic partnerships worldwide, aiming for extensive commercial growth and deeper market reach. With an expansive portfolio of over 20 mRNA-based therapeutic candidates aimed at treating a variety of diseases, WestGene is well-positioned to transform the biopharmaceutical industry.
Advancements at WestGene have been especially notable in three critical areas of mRNA pharmaceutical development: mRNA sequence creation, delivery systems, and production processes. Moreover, patents related to ionizable lipids have been secured across several major markets, including China, the United States, and Europe.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
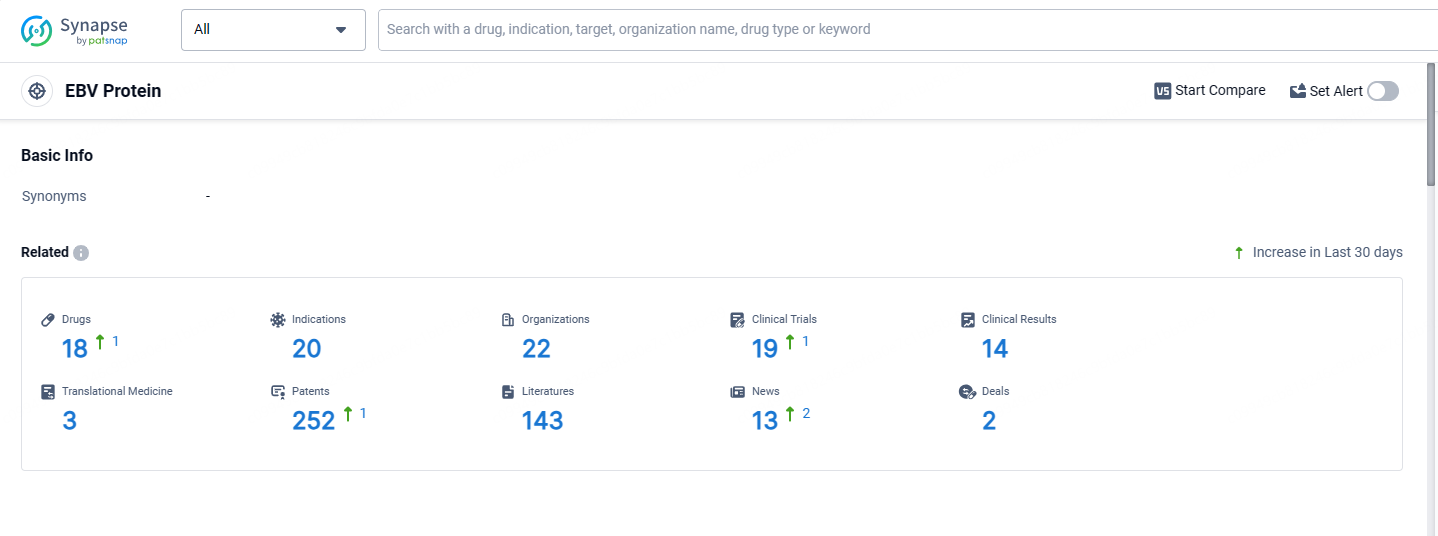 According to the data provided by the Synapse Database, As of May 13, 2024, there are 18 investigational drugs for the EBV protein targets, including 20 indications, 22 R&D institutions involved, with related clinical trials reaching 19, and as many as 252 patents.
According to the data provided by the Synapse Database, As of May 13, 2024, there are 18 investigational drugs for the EBV protein targets, including 20 indications, 22 R&D institutions involved, with related clinical trials reaching 19, and as many as 252 patents.
WGc-043 targets the EBV protein and has potential applications in treating Epstein-Barr virus associated neoplasms, solid tumors, and infections. While it has reached Phase 1 of clinical development, further research is needed to evaluate its effectiveness and safety.