Shionogi & Co. and Maze Therapeutics Announce Global Licensing Deal for Innovative Pompe Disease Therapy, MZE001
Shionogi & Co., Ltd. has entered into an exclusive global licensing agreement with Maze Therapeutics, Inc., securing the rights to MZE001. This investigational drug, an oral inhibitor of glycogen synthase 1 (GYS1), is designed to treat Pompe disease by reducing the accumulation of glycogen that contributes to the disease.
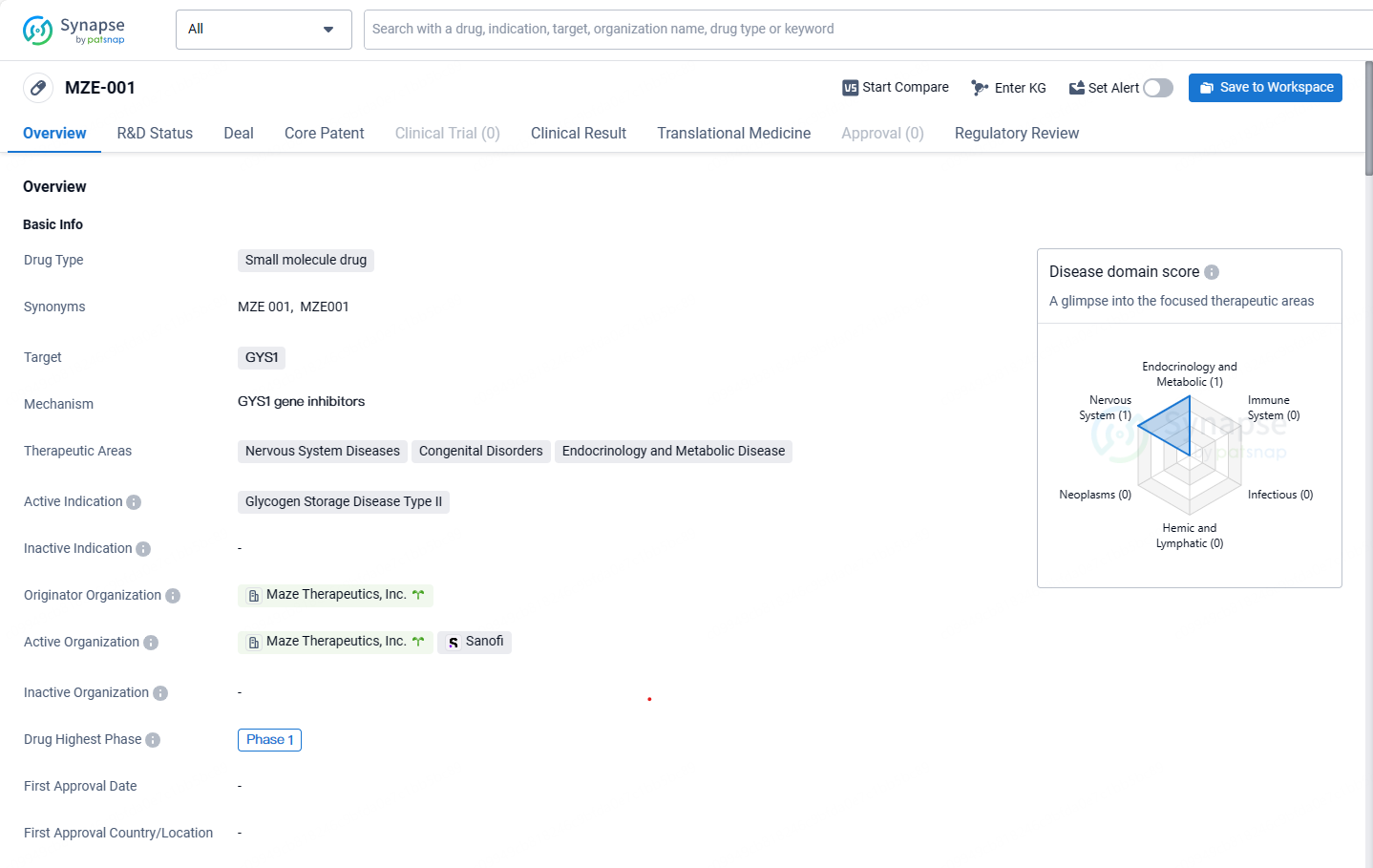
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Pompe disease is an uncommon genetic condition triggered by mutations in the gene responsible for encoding acid alpha-glucosidase. This mutation can cause an accumulation of glycogen in tissues like skeletal muscle, respiratory muscle, and cardiac muscle, leading to progressive muscle weakness and respiratory issues.
Shionogi has secured exclusive global rights for MZE001, in addition to related initiatives and intellectual properties under a newly signed agreement. Shionogi will provide an initial payment of $150 million to Maze, with additional milestone payments contingent on development, regulatory, and sales achievements, plus graduated royalties on future net sales. Following the expiration of the mandatory 30-day waiting period required by the United States Hart-Scott-Rodino Act, this deal has been finalized.
“Entering into this agreement aligns perfectly with Shionogi's strategy. It significantly propels our efforts to create groundbreaking therapies that fulfill critical health needs, enhancing our robust pipeline which is integral to our Medium-Term Business Plan STS2030 Revision,” remarked Isao Teshirogi, Ph.D., CEO of Shionogi.
MZE001 is a selective inhibitor of GYS1, a key enzyme in glycogen synthesis, and is formulated as a small molecule. By obstructing this enzyme's activity, MZE001 lowers glycogen levels in muscle tissues. Preliminary data from the Phase 1 trial of MZE001 indicate its potential as the pioneering oral treatment for Pompe disease. MZE001 might be administered both as stand-alone therapy and together with enzyme replacement therapies, the established treatment standard, to potentially enhance patient outcomes in Pompe disease.
“Shionogi is invested in both advancing and launching MZE001, recognizing the profound impact it could have on patients and their pressing health requirements,” stated Jason Coloma, Ph.D., CEO of Maze. “With an established history of developing and providing innovative treatments globally, Shionogi is ideally suited to propel MZE001 through the necessary clinical trials to quickly bring relief to individuals battling this severe disease.”
In 2022, MZE001 received Orphan Drug Designation from the U.S. Food and Drug Administration. This designation is granted to facilitate the development of drugs that target rare diseases or conditions. Benefits under the Orphan Drug Act include tax rebates, funding for clinical testing, a waiver for some fees, and exclusive marketing rights for seven years post-approval.
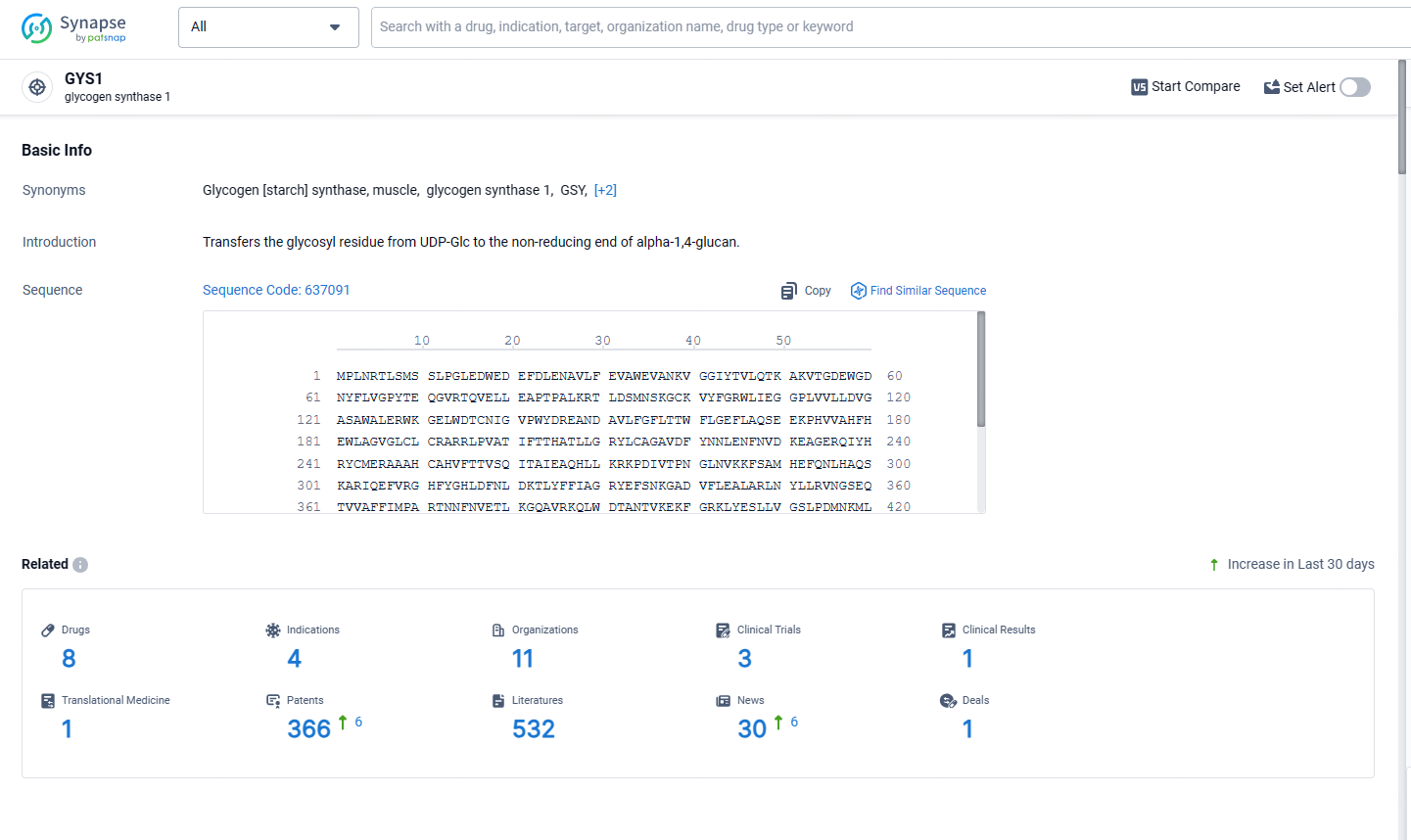
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of May 13, 2024, there are 8 investigational drugs for the GYS1 targets, including 4 indications, 11 R&D institutions involved, with related clinical trials reaching 3, and as many as 366 patents.
MZE-001 targets the GYS1 protein and is currently in Phase 1 of clinical development. The drug has been designated as an orphan drug, indicating its potential to address an unmet medical need in a rare disease population.