FDA Greenlights Aprea's Novel WEE1 Kinase Inhibitor APR-1051 for Cancer Trial
Aprea Therapeutics, Inc., an enterprise in the biopharmaceutical sector that is at the clinical-phase and emphasizes targeted cancer treatments via synthetic lethality, has made a public statement confirming that their application for an Investigational New Drug (IND), specifically for APR-1051, has received approval from the U.S. Food and Drug Administration.
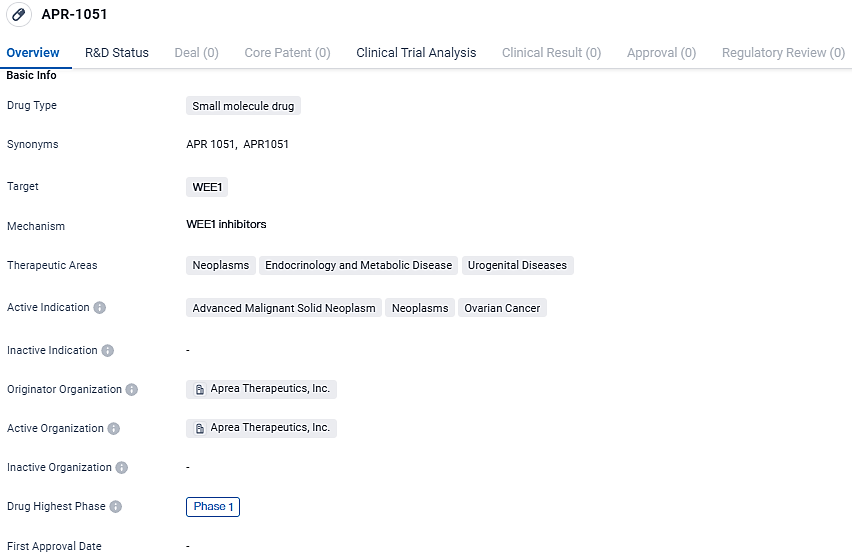
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"APR-1051 represents an advanced inhibitor specifically targeting WEE1 kinase, and with its distinct attributes, we are optimistic about it setting the standard in its category," proclaimed Dr. Oren Gilad, the CEO of Aprea.
Elaborating further, Dr. Gilad noted, "With the FDA granting approval for our Investigational New Drug application, this marks a pivotal moment for the advancement of APR-1051. Our team is eager to commence the exploration of its efficacy in treating patients, particularly those with cancers characterized by high levels of Cyclin E, such as ovarian and breast cancers."
Aprea's dedicated team of chemists and researchers were responsible for the discovery and initial tests of APR-1051. The company has completed a battery of preclinical assessments, revealing the compound's strong capabilities in combating tumors and possessing a beneficial pharmacokinetic (PK) profile. Moreover, the preliminary data suggests that APR-1051 could have a reduced toxicity profile when compared to existing WEE1 kinase inhibitors.
The go-ahead for the IND application paves the way for Aprea to begin the Phase 1 ACESOT-1051 dose escalation trial. This trial is intended to gather data on APR-1051's safety, tolerability, and initial effectiveness. The milestone of enrolling the initial participant for this study is projected for the earlier part of 2024, with progress updates anticipated by the end of that year.
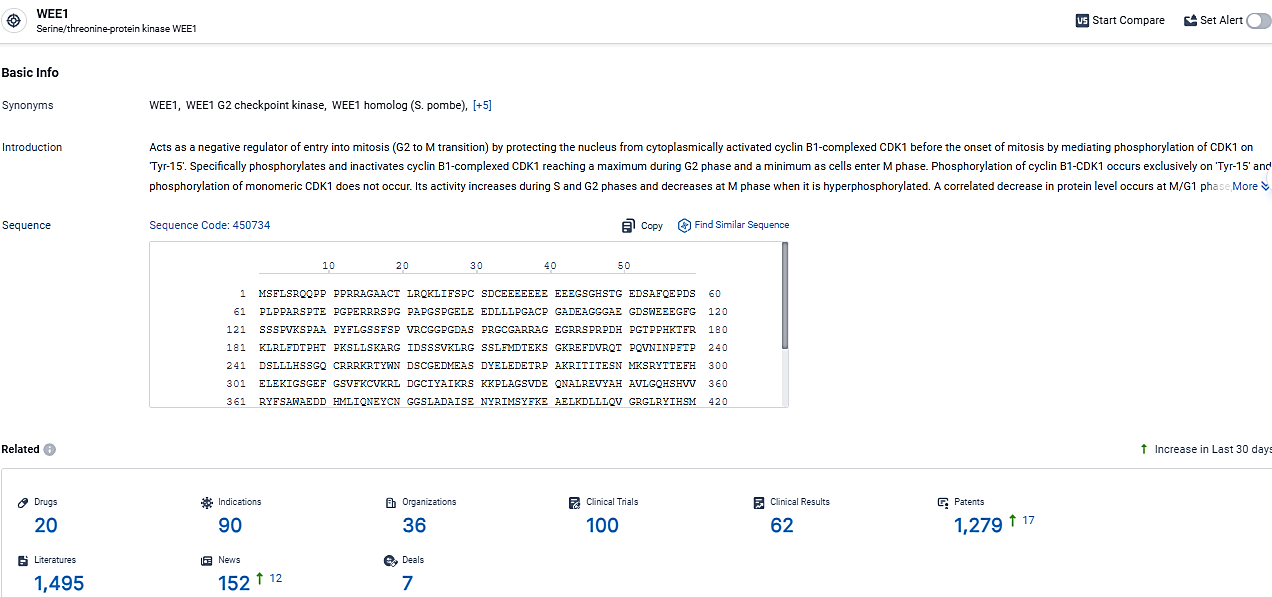
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of March 12, 2024, there are 20 investigational drugs for the WEE1 target, including 90 indications, 36 R&D institutions involved, with related clinical trials reaching 100, and as many as 1279 patents.
APR-1051 targets WEE1 and is being developed for the treatment of advanced malignant solid neoplasms, neoplasms, and ovarian cancer. With its potential applications in neoplasms, endocrinology and metabolic disease, and urogenital diseases, APR-1051 shows promise in the field of biomedicine. However, as it is currently in Phase 1, further research and clinical trials are needed to establish its safety and efficacy.