AbilityPharma Receives €7M for Phase 2b Trials of Cancer Drug ABTL0812
The biotech firm from Catalonia, Ability Pharmaceuticals, SA, which is dedicated to crafting novel anticancer drugs that stimulate autophagy via oral administration, has declared the procurement of a €7 million capital infusion. This investment comes from a consortium of investors with ties to life sciences sectors based in Europe and Canada. Prominent contributors to this funding round encompass CTI Life Sciences Fund, Inveready, the EIC Fund, Fitalent, and CDTI Innvierte.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
This capital injection will enable the enterprise to allocate full resources towards the completion of its Phase 2b trial concerning the cancer-inhibiting agent ABTL0812 among individuals diagnosed with pancreatic cancer.
At present, AbilityPharma is advancing a Phase 2b study utilizing ABTL0812 to address metastatic pancreatic cancer, endeavoring to surpass the efficacy of the established therapy regimen FOLFIRINOX. This rigorous, randomized, and placebo-controlled initial treatment study intertwines with FOLFIRINOX and takes place across 23 medical institutions based in the United States, Spain, France, and Israel. Anticipated effectiveness outcomes are slated for disclosure by the culmination of this year.
Outcomes indicating markedly enhanced effectiveness will position AbilityPharma favorably to secure capital for ABTL0812's conclusive development phase or to initiate licensing negotiations with a global entity in the pharmaceutical or biotechnological sector, aiming to disperse ABTL0812 to those affected by pancreatic cancer by the year 2028.
With enthusiasm, Carles Domènech, PhD, who serves as the Executive Chairman and CEO of AbilityPharma, expressed, "Securing this round of capital is a major milestone for us, and we're excited to have Shermaine Tilley of CTI Life Sciences Fund join our board, a move that will help us expedite ABTL0812's development efforts promptly."
Carles Domènech further noted his appreciation: "Gratitude is extended to CTI Life Sciences, Inveready, and the EIC Fund for their role in closing this investment round. Our profound thanks to both new and continuing supporters for their trust in our team and their endorsement of our mission. Their financial commitment empowers us to relentlessly push forward in delivering ABTL0812 to those grappling with pancreatic cancer."
Shermaine Tilley, PhD, MBA, and Managing Partner at CTI Life Sciences Fund, shared her contentment by saying, “Supporting Ability Pharma in the advanced stages of their groundbreaking treatment for pancreatic cancer fills us with pride. Accepting a role on the board, I am keen to collaborate closely with both management and fellow board members to maximize ABTL0812's potential benefits for stakeholders and patients alike.”
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
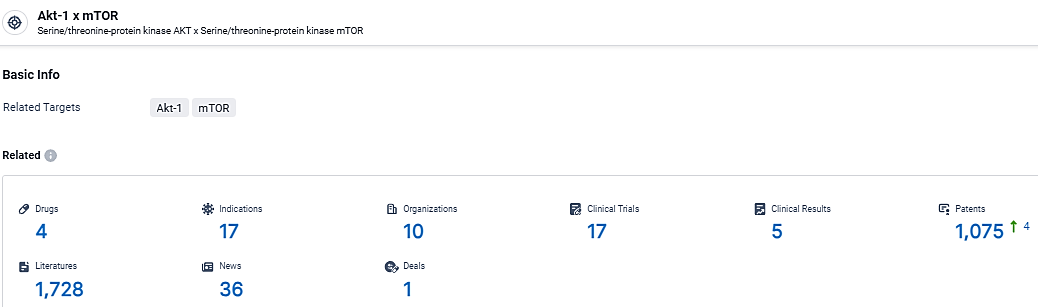
According to the data provided by the Synapse Database, As of March 12, 2024, there are 4 investigational drugs for the Akt-1 x mTOR target, including 17 indications, 10 R&D institutions involved, with related clinical trials reaching 17, and as many as 1075 patents.
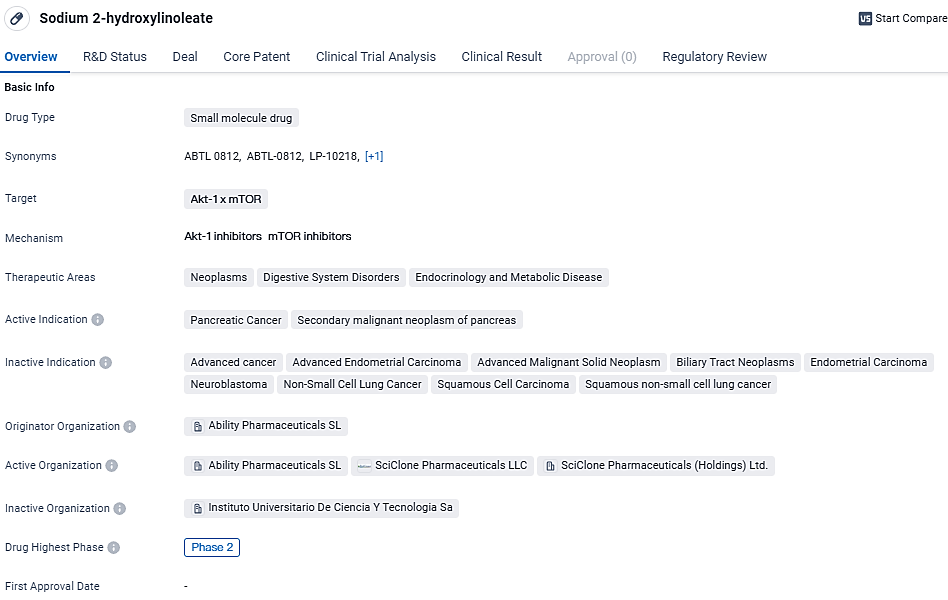
ABTL0812 is a small molecule drug currently in Phase 2 of development. It targets Akt-1 x mTOR and has potential therapeutic applications in neoplasms, digestive system disorders, and endocrinology and metabolic diseases. Its active indications are focused on pancreatic cancer and secondary malignant neoplasms of the pancreas. The drug has received orphan drug designation and is part of a special review project. Further research and regulatory processes are required to fully evaluate its potential in treating these conditions.