FDA Prioritizes GSK's Proposal to Expand Jemperli Use with Chemotherapy in Advanced Endometrial Cancer
GSK plc revealed that the US Food and Drug Administration has approved the revised Biologics License Application for Jemperli (dostarlimab) used alongside standard-of-care chemotherapy drugs, carboplatin and paclitaxel, aimed at broadening its use to include all adult individuals suffering from primary advanced or recurrent endometrial cancer. The expansion covers patients with mismatch repair proficient (MMRp) or microsatellite stable (MSS) cancers.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Jemperli has received FDA approval for use in combination with carboplatin and paclitaxel, followed by its administration as a stand-alone therapy for adult patients suffering from primary advanced or recurrent endometrial cancer that is categorized as mismatch repair deficient or has high microsatellite instability, as verified by an FDA-approved test.
This application was given Priority Review status by the FDA, and a decision under the Prescription Drug User Fee Act is expected by 23 August 2024.
This supplemental Biologics License Application (sBLA) draws upon data from the initial portion of the RUBY phase III clinical study. In this trial, the primary goals related to progression-free survival and overall survival were achieved, showing a significant and clinically important advantage for patients receiving a combination of dostarlimab with carboplatin-paclitaxel in comparison to those undergoing chemotherapy alone.
Part 1 of the RUBY trial remains the sole clinical study to demonstrate a statistically significant improvement in survival across the entire group of participants. The examination of safety and tolerance within the RUBY trial indicated that the combination of dostarlimab with carboplatin-paclitaxel had a safety profile aligning with those observed for each agent independently.
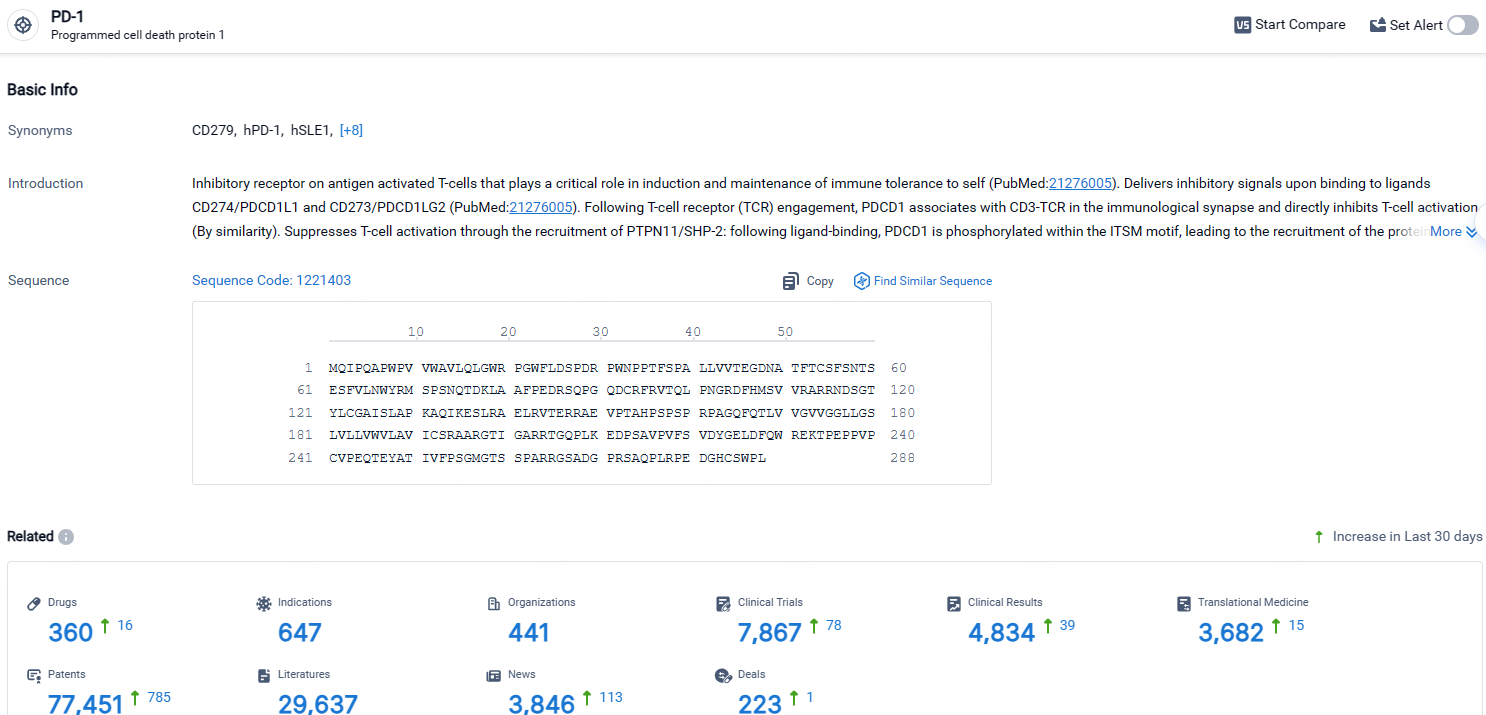
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of April 28, 2024, there are 360 investigational drugs for the PD-1 target, including 647 indications, 441 R&D institutions involved, with related clinical trials reaching 7867, and as many as 77451 patents.
Dostarlimab-gxly is a monoclonal antibody drug that targets PD-1 and has been approved for the treatment of a wide range of neoplastic and non-neoplastic diseases. Its approval in multiple countries and the utilization of various regulatory pathways highlight its potential as a significant therapeutic option in the field of biomedicine.