Ferring Presents Early Results from Phase 3 Trial of SI-6603 for Lumbar Disc Herniation at ASPN 2024
Ferring Pharmaceuticals, in collaboration with their clinical development partner Seikagaku Corporation, has shared the findings of a Phase 3 data analysis on the early treatment response to SI-6603 (generic name: condoliase). This investigational therapy is being explored for the management of radicular leg pain caused by lumbar disc herniation. The results were unveiled at the American Society of Pain and Neuroscience Annual Meeting and encompassed an examination of healthcare resource usage and the expenses related to LDH surgery.
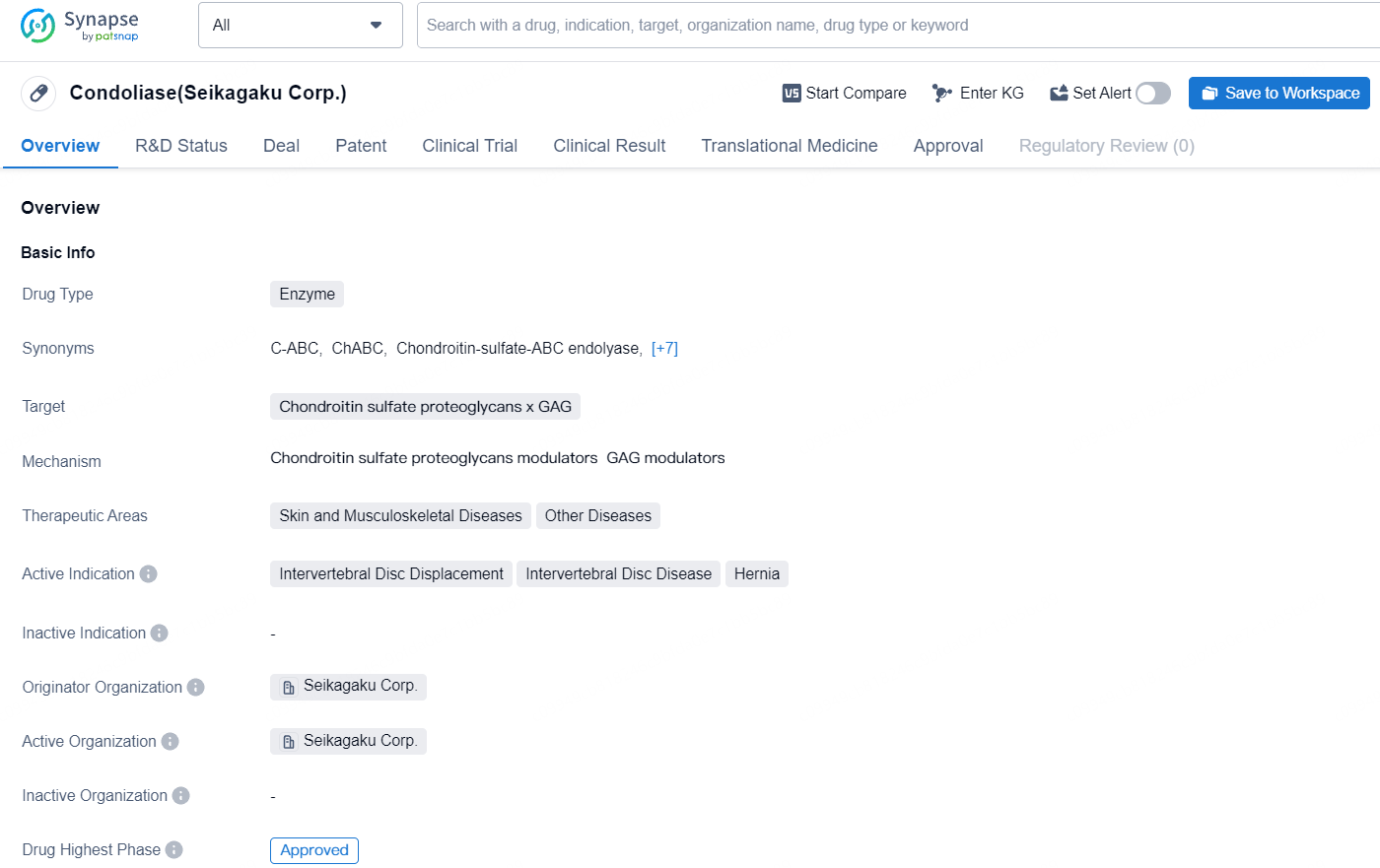
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
In the evaluation of the double-blind, sham-controlled, Phase 3 trial, participants with lumbar disc herniation (LDH) were assigned either a single intradiscal injection of SI-6603 at a dosage of 1.25 units or a placebo injection, followed by a 52-week observation period. The modified intention-to-treat (mITT) cohort comprised 341 individuals.
“The early therapeutic response seen in this study appears promising for patients suffering from radicular leg pain due to lumbar disc herniation who endure severe, debilitating pain that can limit their mobility and daily activities. This represents a significant area of unmet patient need. If approved, SI-6603, which directly targets the source of radicular leg pain, could potentially bridge the current gap between conservative pain management and surgical intervention,” stated Dr. Raza Ahmed, Senior Director, Medical Affairs, Specialty/Orthopaedics, Ferring USA.
An analysis of healthcare resource utilization and costs associated with surgical procedures for LDH patients was also presented at the ASPN 2024 conference. From the medical claims database, out of over one million patients with LDH, 58,328 underwent surgeries related to LDH within a year of their initial diagnosis.
The trial investigated U.S. participants aged 30 to 70 years with contained posterolateral LDH, primarily exhibiting unilateral radiculopathy/radicular leg pain and inadequate pain relief despite more than six weeks of conservative treatment. Of the 352 randomized individuals, 341 were included in the mITT cohort.
SI-6603, containing condoliase as the active ingredient, is an experimental treatment aimed at reducing radicular leg pain associated with lumbar disc herniation through a single, direct intradiscal injection. SI-6603 functions by decreasing nerve root compression, thus alleviating pain.
SI-6603 was developed by Seikagaku Corporation. It received marketing approval in Japan from the Ministry of Health, Labour, and Welfare in March 2018, and has been distributed exclusively in Japan as HERNICORE 1.25 units for intradiscal injection by Seikagaku Corporation's sales partner, Kaken Pharmaceutical Co., Ltd., since August 1, 2018.
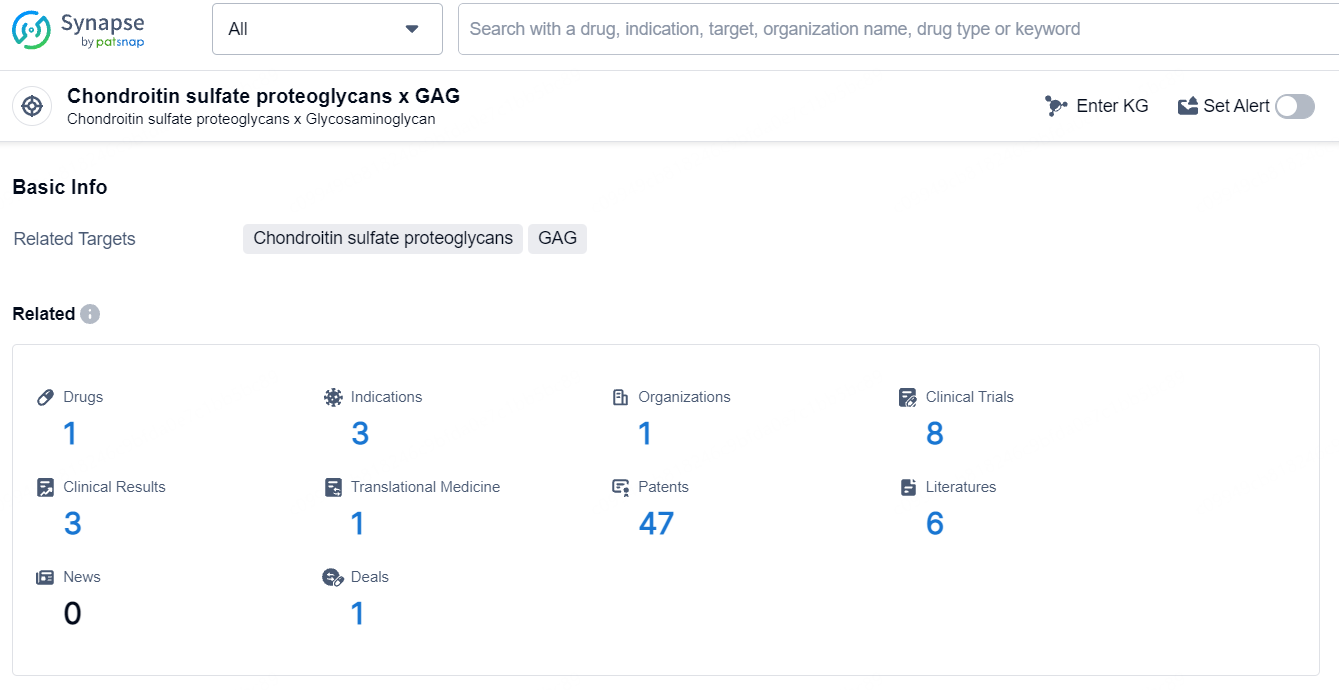
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of July 17, 2024, there are 1 investigational drug for the chondroitin sulfate proteoglycans and GAG targets, including 3 indications, 1 R&D institution involved, with related clinical trials reaching 8, and as many as 47 patents.
Condoliase is an enzyme-based drug targetingchondroitin sulfate proteoglycans x GAG to treat a range of conditions in the therapeutic areas of skin and musculoskeletal diseases, as well as other diseases. Condoliase represents a significant advancement in the pharmaceutical industry's efforts to develop innovative treatments for challenging conditions like intervertebral disc displacement and disease. Its approval in Japan marks an important milestone in the drug's journey towards potentially becoming available in other countries and making a positive impact on patients' lives worldwide.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!