Is Celecoxib/Tramadol approved by the FDA?
Yes, celecoxib and tramadol, marketed under the brand name Seglentis, received FDA approval on October 15, 2021. This combination medication is used in adults for the management of pain severe enough to require an opioid analgesic and for which alternative treatments are inadequate.
What is Celecoxib/Tramadol?
Celecoxib is a nonsteroidal anti-inflammatory drug (NSAID) that helps reduce pain and inflammation. Tramadol is an opioid pain medication that acts on the central nervous system to relieve pain. When combined, these two drugs offer a potent solution for managing acute pain.
Usage
Celecoxib and tramadol are used when other pain medications are ineffective or cannot be used. This combination can manage acute pain severe enough to require an opioid analgesic. It is taken orally, usually in the form of a tablet containing 56 mg of celecoxib and 44 mg of tramadol.
Dosage and Administration
The typical dosage for adults is two tablets taken orally every 12 hours as needed for pain. It is important to follow the prescription guidelines closely to avoid the risk of serious side effects, including respiratory depression.
Warnings and Precautions
- Addiction and Misuse: Misuse of celecoxib and tramadol can lead to addiction, overdose, or death. It is crucial to store this medication where others cannot access it.
- Cardiovascular Risks: This combination can increase the risk of fatal heart attack or stroke, even in those without pre-existing risk factors. It should not be used before or after heart bypass surgery.
- Gastrointestinal Risks: Celecoxib and tramadol may cause stomach or intestinal bleeding, which can be fatal and occur without warning, particularly in older adults.
Side Effects
Serious Side Effects
Seek immediate medical attention if you experience any of the following:
- Signs of an allergic reaction (hives, difficulty breathing, swelling of the face or throat)
- Serotonin syndrome symptoms (agitation, hallucinations, fever, sweating, shivering, fast heart rate, muscle stiffness, twitching, loss of coordination, nausea, vomiting, or diarrhea)
- Severe skin reactions (fever, sore throat, burning eyes, skin pain, red or purple skin rash with blistering and peeling)
- Respiratory issues (slow or stopped breathing)
- Symptoms of a heart attack or stroke (chest pain spreading to your jaw or shoulder, sudden numbness or weakness on one side of the body, slurred speech, leg swelling, shortness of breath)
- Kidney problems (little or no urination, swelling in the feet or ankles, feeling tired or short of breath)
- Liver problems (nausea, upper right stomach pain, itching, tiredness, dark urine, jaundice)
Common Side Effects
- Nausea
- Vomiting
- Dizziness
- Headache
- Drowsiness
Drug Interactions
Celecoxib and tramadol can interact with many medications, including antidepressants, benzodiazepines, other NSAIDs, other opioids, and medications to treat or prevent blood clots. Always inform your doctor about all the medications you are taking to avoid potentially dangerous interactions.
Storage and Disposal
Store celecoxib and tramadol at room temperature, away from moisture and heat. Keep track of your medication and dispose of any leftover medicine through a drug take-back program or by mixing it with cat litter or coffee grounds in a sealed plastic bag and throwing it in the trash.
Conclusion
Celecoxib and tramadol (Seglentis) is a powerful combination for managing severe pain when other treatments are insufficient. Approved by the FDA on October 15, 2021, it combines the anti-inflammatory properties of celecoxib with the pain-relieving effects of tramadol. It is crucial to follow the prescribed dosage and guidelines closely, be aware of potential side effects, and communicate with your healthcare provider about any other medications you are taking to ensure safe and effective use.
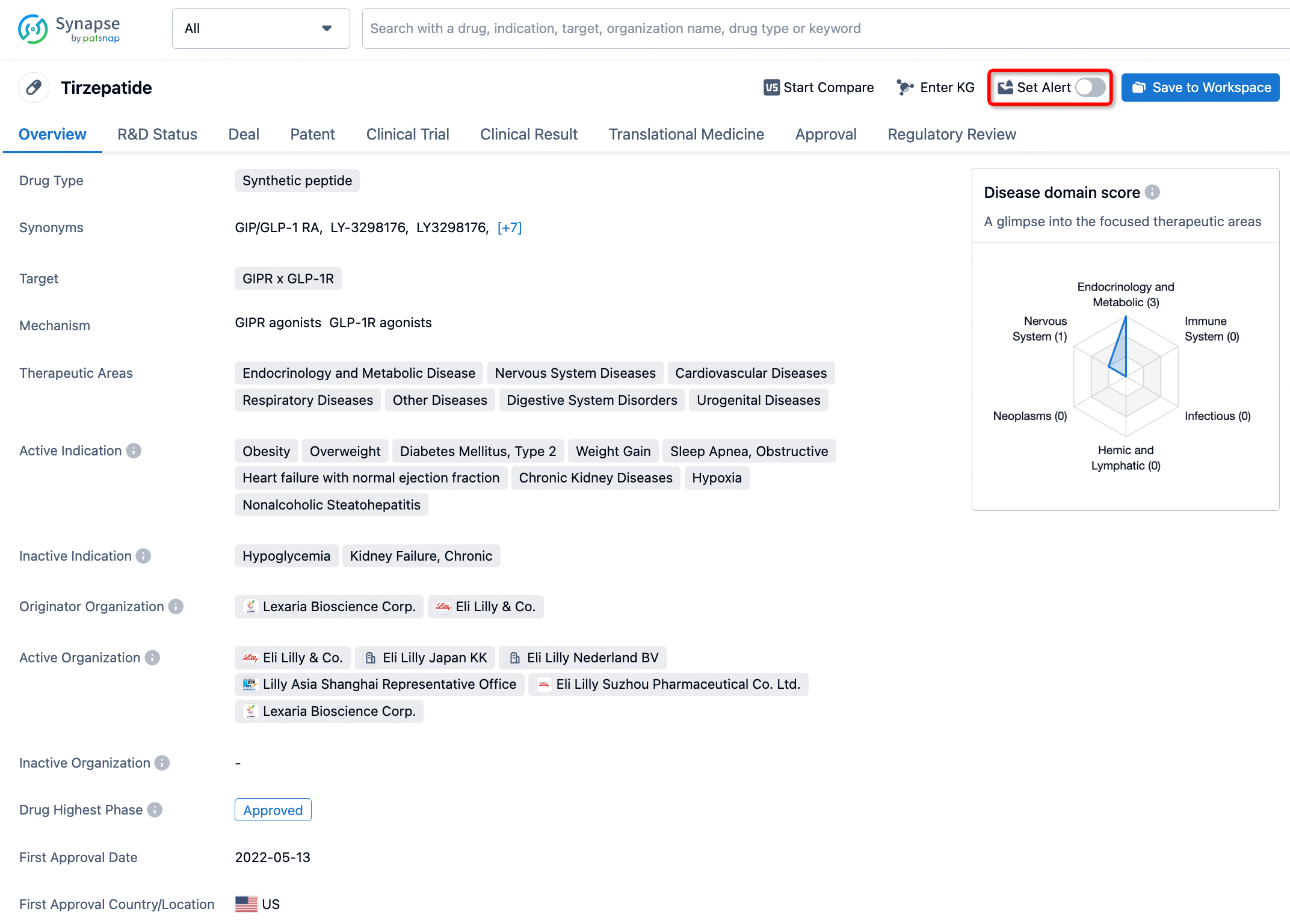
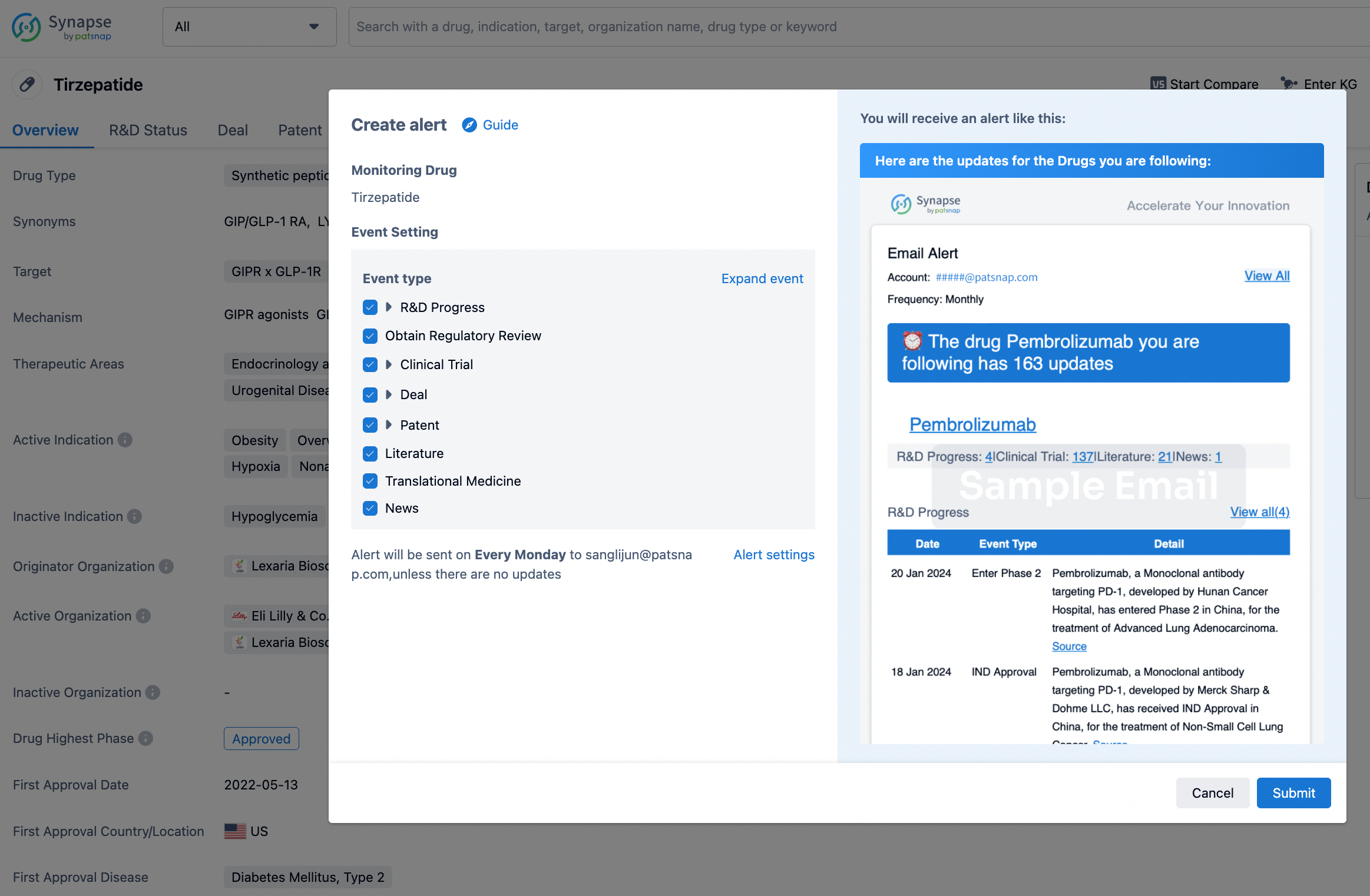
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!