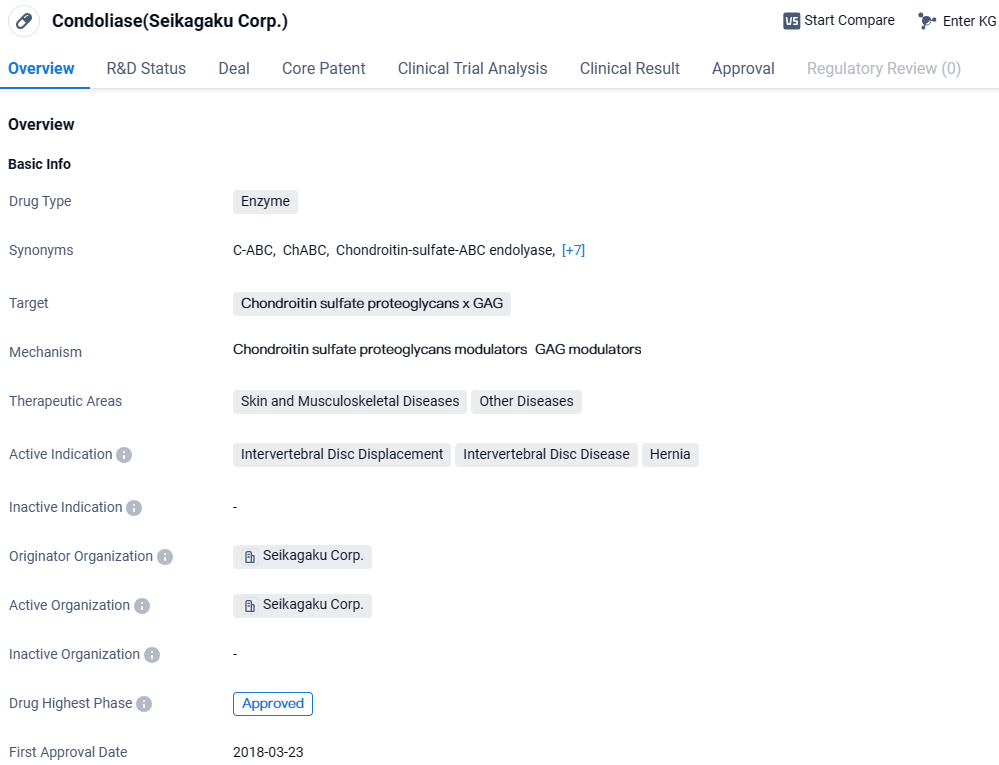
Ferring Presents Phase 3 Findings for SI-6603 in Back Disc Protrusion at ASIPP 2024
Ferring Pharmaceuticals, in collaboration with its clinical research associate, Seikagaku Corporation, has unveiled research findings examining the effectiveness and safety parameters of SI-6603 (generic name: condoliase), a product under investigation aimed at managing symptomatic leg pain linked to herniated lumbar discs.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The study findings were showcased at the annual conference for the American Society of Interventional Pain Physicians. The investigation embraced a pivotal Phase 3 study and a comprehensive safety evaluation drawn from six clinical trials, in conjunction with a pragmatic examination of the existing therapeutic methodologies and the observable shortfalls in the medical treatment of individuals who have been recently diagnosed with LDH.
"Lumbar disc herniation stands as the principal instigator of sciatica, provoking intense pain that impairs mobility and deteriorates the standard of living, potentially necessitating a surgical remedy," stated Raza Ahmed, MD, who is a Senior Director at Ferring USA overseeing Medical Affairs in Specialty and Orthopedic divisions. "The information we've gathered marks an encouraging stride towards offering patients a less intrusive therapeutic alternative for this grave health issue."
Kenneth Candido, MD, affiliated with Chicago Anesthesia Pain Specialists and holding a Clinical Professorship in Anesthesia & Surgery at the University of Illinois in Chicago, IL, noted, "For an extended period, the management of radicular leg discomfort due to LDH has been confined to non-aggressive treatment involving analgesics and physical rehabilitation, or going the route of advanced surgical procedures." He added, "In light of our discoveries, SI-6603 emerges as a potential treatment avenue, wherein a sole intradiscal administration could grant considerable alleviation from the persistent torment frequently linked with this condition."
SI-6603 is a developmental treatment featuring condoliase as the principal active ingredient and is tailored to mitigate radicular pain in the legs tied to LDH through a one-time injection directly into the affected intervertebral disc.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
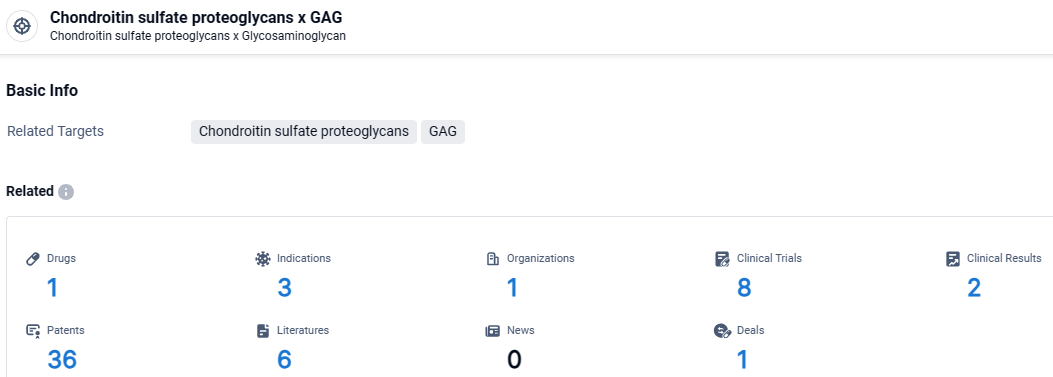
According to the data provided by the Synapse Database, As of April 9, 2024, there are 1 investigational drugs for the Chondroitin sulfate proteoglycans and GAG target, including 3 indications, 1 R&D institutions involved, with related clinical trials reaching 8, and as many as 36 patents.
Condoliase targets chondroitin sulfate proteoglycans and is approved for use in treating intervertebral disc displacement, intervertebral disc disease, and hernia. The drug has its first approval in Japan and is primarily used in the therapeutic areas of skin and musculoskeletal diseases, as well as other diseases.