FluoGuide reports positive initial results from FG001 Phase IIa head and neck cancer study at International Academy of Oral Oncology meeting
FluoGuide A/S, a leader in the forefront discipline of precision oncology surgery, revealed that preliminary outcomes from the phase IIa study for its prominent product, FG001, in directing surgical procedures on patients with head and neck malignancies were shared at the International Academy of Oral Oncology 2023 conference in Incheon, South Korea. The study confirmed the product's positive impacts and safety.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"The initial uplifting clinical outcomes, which demonstrated FG001's capability to illuminate tumors in all head & neck cancer patients involved in the experiment, suggest it could be of significant benefit to operating surgeons and the individuals they treat.
The collected data points towards various possible uses in the context of head & neck cancer, and I'm eager to observe more results deriving from FG001’s clinical progression,” stated Anders Christensen, MD, PhD, the Chief Investigator and head and neck surgeon, who showcased the data at IAOO. FG001 serves as a fluorophore targeting uPAR, a cancer-specific marker found abundantly in most solid tumour types.
It is injected into a patient’s vein before surgery and illuminates the cancer during the procedure, assisting the surgeon in eradicating all the cancerous cells while preserving healthy tissue. The fluorophore shares the same spectral attributes as indocyanine green, already approved, thus signifying FG001's compatibility with most imaging equipment.
This open-label, mono-centric, non-randomized, exploratory phase lla experiment examined the impact of FG001 in directing surgical procedures of patients ailing from head & neck cancer. The objective was to secure proof-of-concept for the uPAR platform technology intended to aid in the surgical excision of cancer.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
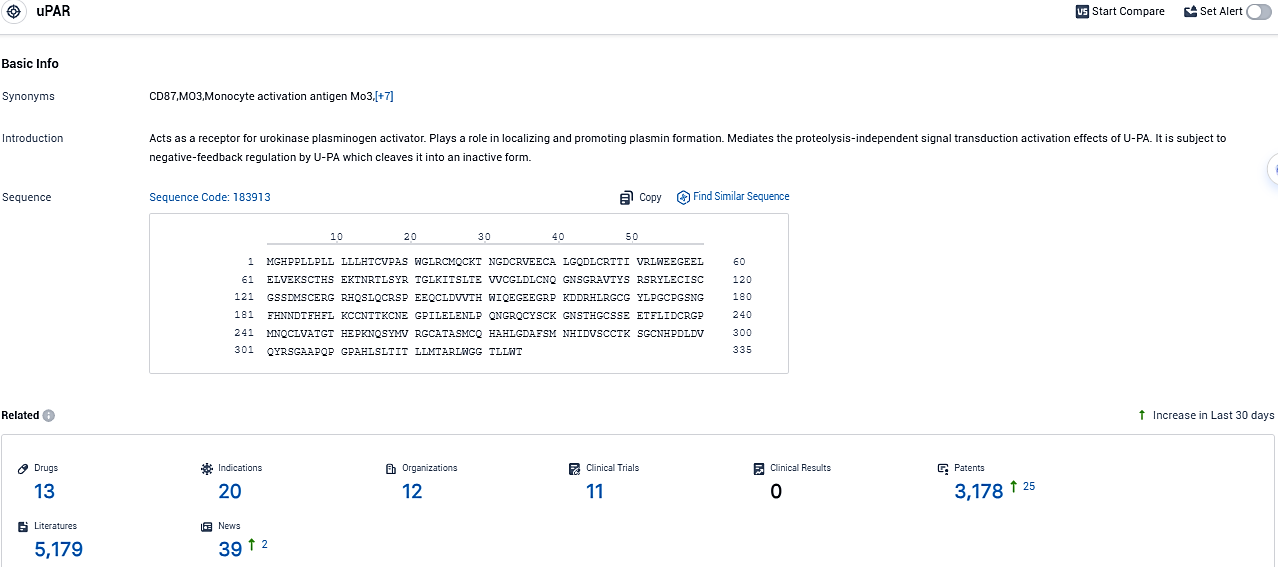
According to the data provided by the Synapse Database, As of November 14, 2023, there are 13 investigational drugs for the uPAR target, including 20 indications, 12 R&D institutions involved, with related clinical trials reaching 11, and as many as 3178 patents.
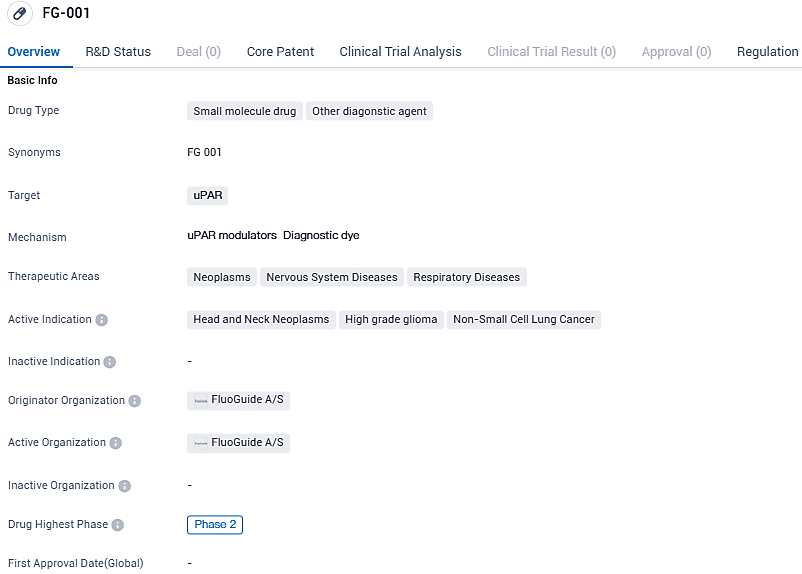
FG-001 targets uPAR and is being developed for the treatment of neoplasms, nervous system diseases, and respiratory diseases. The active indications for FG-001 include head and neck neoplasms, high-grade glioma, and non-small cell lung cancer. FluoGuide A/S is currently conducting Phase 2 clinical trials for FG-001, and the drug has been granted orphan drug status.