MoonLake Immunotherapeutics reveals significant outcomes from Phase 2 trials of the Nanobody® medication, sonelokimab, for ongoing psoriatic arthritis
MoonLake Immunotherapeutics, a biotech firm at the clinical-stage dedicated to developing advanced therapies for inflammatory conditions, has revealed promising preliminary outcomes from its worldwide Phase 2 ARGO trial. This trial was designed to examine the effectiveness and security of Nanobody® sonelokimab in individuals suffering from active psoriatic arthritis.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In the ARGO study, which engaged 207 patients, it fulfilled its primary target by recording a statistically substantial higher rate of patients being treated with either sonelokimab 60mg or 120mg first achieving an American College of Rheumatology 50 response over those who received a placebo at the 12th week.
Specifically, 46% and 47% of sonelokimab-treated patients achieved ACR50 for 60mg and 120mg doses given with induction correspondingly, with a significant difference over placebo. Moreover, 78% and 72% reached ACR20 while 29% and 26% achieved ACR70. The main analyses were executed on the strictest analysis type for trials of this kind, namely, intention-to-treat with non-responders included.
As anticipated, the 60mg dose without induction did not reach statistical significance, validating the probable efficacy of the 60mg and 120mg doses with induction to be progressed into Phase 3. All crucial secondary targets were achieved for the 60mg and 120mg doses administered with induction.
The main supplementary endpoint, Psoriasis Area and Severity Index 90, was met for all doses provided with induction, with 77% of patients responding at week 12 to the 60mg dose. Jorge Santos da Silva, PHD, the founder and CEO of MoonLake said, “Our team is dedicated to improving patient outcomes, and we have set high expectations for our Nanobody® sonelokimab. ARGO is MoonLake’s third second-phase trial and the first to report positive topline results in psoriatic arthritis using a Nanobody®, marking another significant achievement. Our goals were once again met in this PsA trial.”
Adalimumab acted as an active reference to verify responses across arms. Sonelokimab 60mg and 120mg numerically outperformed adalimumab for both primary endpoint and all significant secondary endpoints, reinforcing the promising future potential of sonelokimab as a leading therapeutic approach.
Kristian Reich, MD, PhD, MoonLake’s founder and chief scientific officer, shared his thoughts, "We are confident that we have significantly improved the therapy by achieving momentous clinical outcomes at week 12. The data supports the unique molecular properties of sonelokimab and its IL-17A andIL-17F inhibition ability to efficiently manage inflammation in deep tissues."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
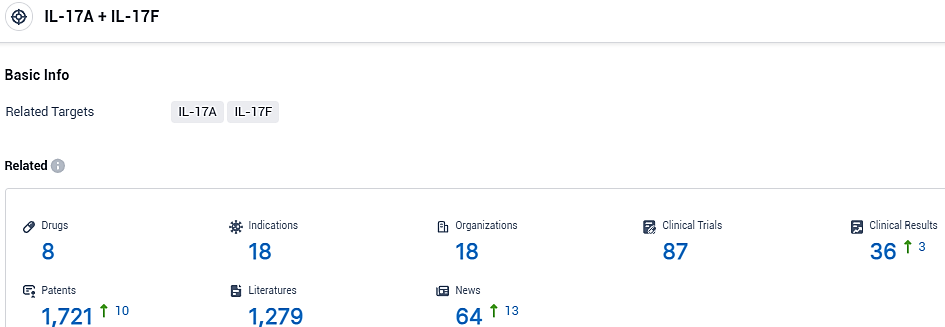
According to the data provided by the Synapse Database, As of November 12, 2023, there are 8 investigational drugs for the IL-17A and IL-17F target, including 18 indications, 18 R&D institutions involved, with related clinical trials reaching 87, and as many as 1721 patents.
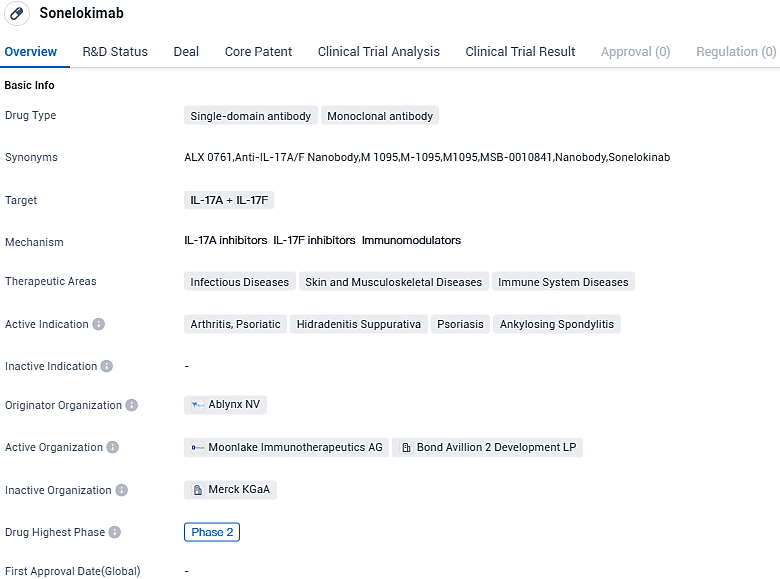
Sonelokimab is a single-domain antibody and monoclonal antibody that targets IL-17A and IL-17F. With its potential to address multiple indications in the field of infectious diseases, skin and musculoskeletal diseases, and immune system diseases, Sonelokimab holds promise as a targeted treatment option for patients suffering from arthritis, psoriatic, hidradenitis suppurativa, psoriasis, and ankylosing spondylitis. Further research and clinical trials will be necessary to determine its safety and efficacy in a larger patient population.