GB-1211: brief review of its R&D progress and the clinical result in 2023 ESMO
On 23 Oct 2023, GALLANT-1: GB1211 galectin-3 (Gal-3) inhibitor plus atezolizumab (atz) for first line treatment in patients (pts) with advanced/metastatic non-small cell lung cancer (NSCLC) was reported at the ESMO Congress.
GB-1211's R&D Progress
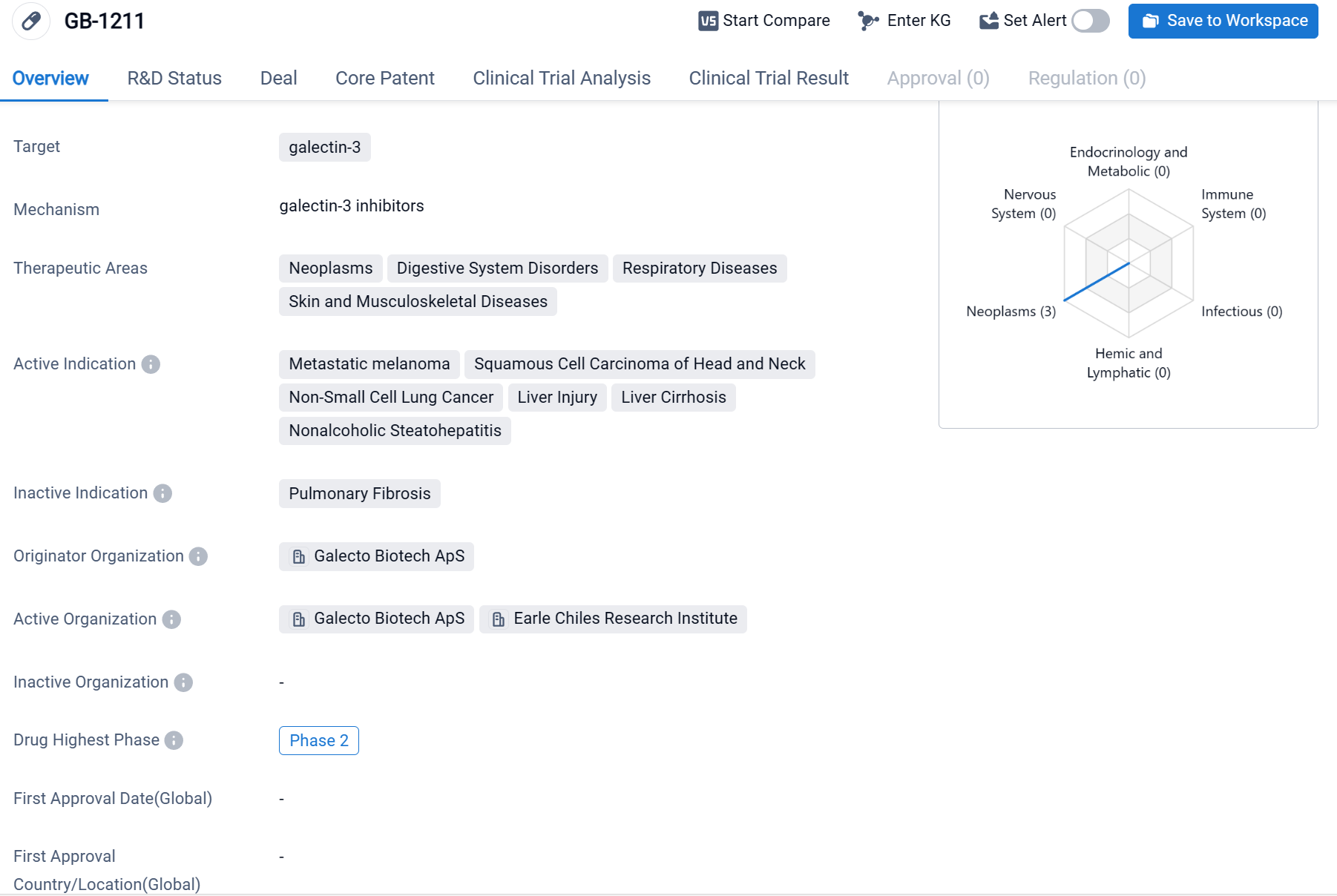
GB-1211 is a small molecule drug that targets galectin-3, a protein involved in various disease processes. It has shown potential therapeutic benefits in multiple therapeutic areas, including neoplasms, digestive system disorders, respiratory diseases, and skin and musculoskeletal diseases.
According to the Patsnap Synapse, as of the latest available information, GB-1211 has reached the highest phase of clinical development, which is Phase 2. And the clinical trial areas for GB-1211 are primarily in Bulgaria. The key indication is Non-Small Cell Lung Cancer. 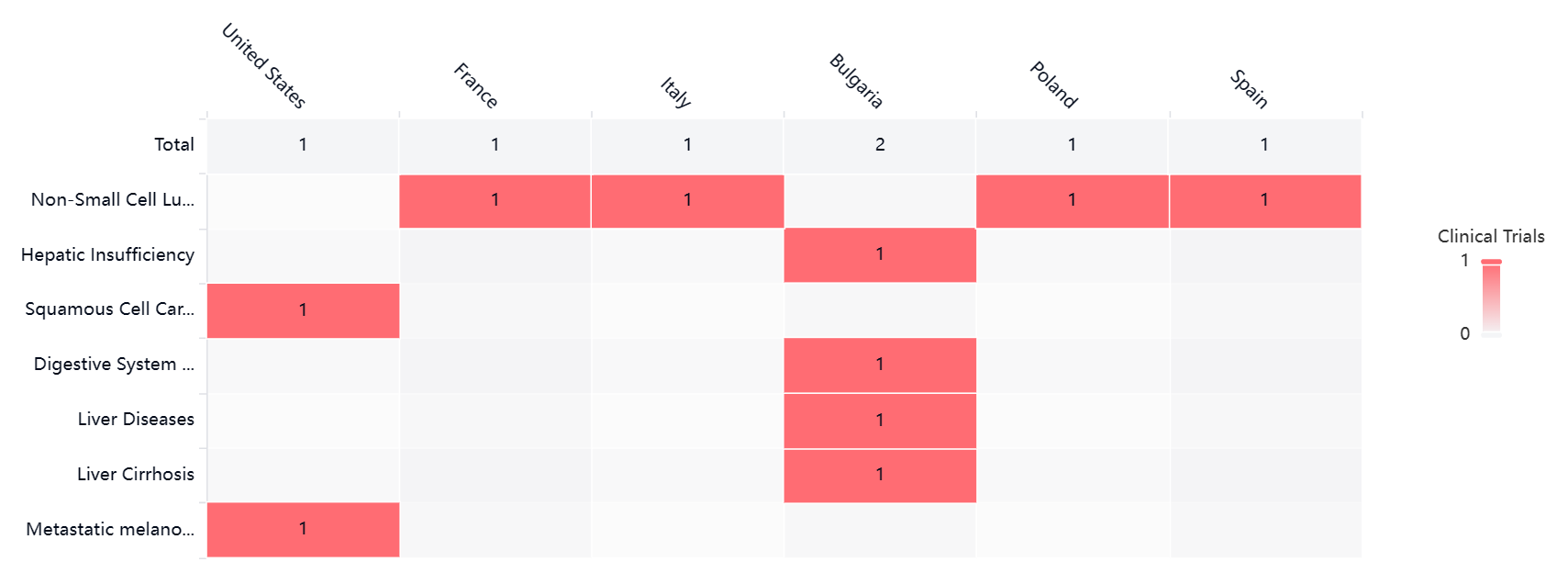
Detailed Clinical Result of GB-1211
The randomized, parallel assignment, quadruple masking clinical trial (NCT05240131) was a dose confirmation study of GB1211 in patients (pts) with advanced/metastatic non-small cell lung cancer (NSCLC).
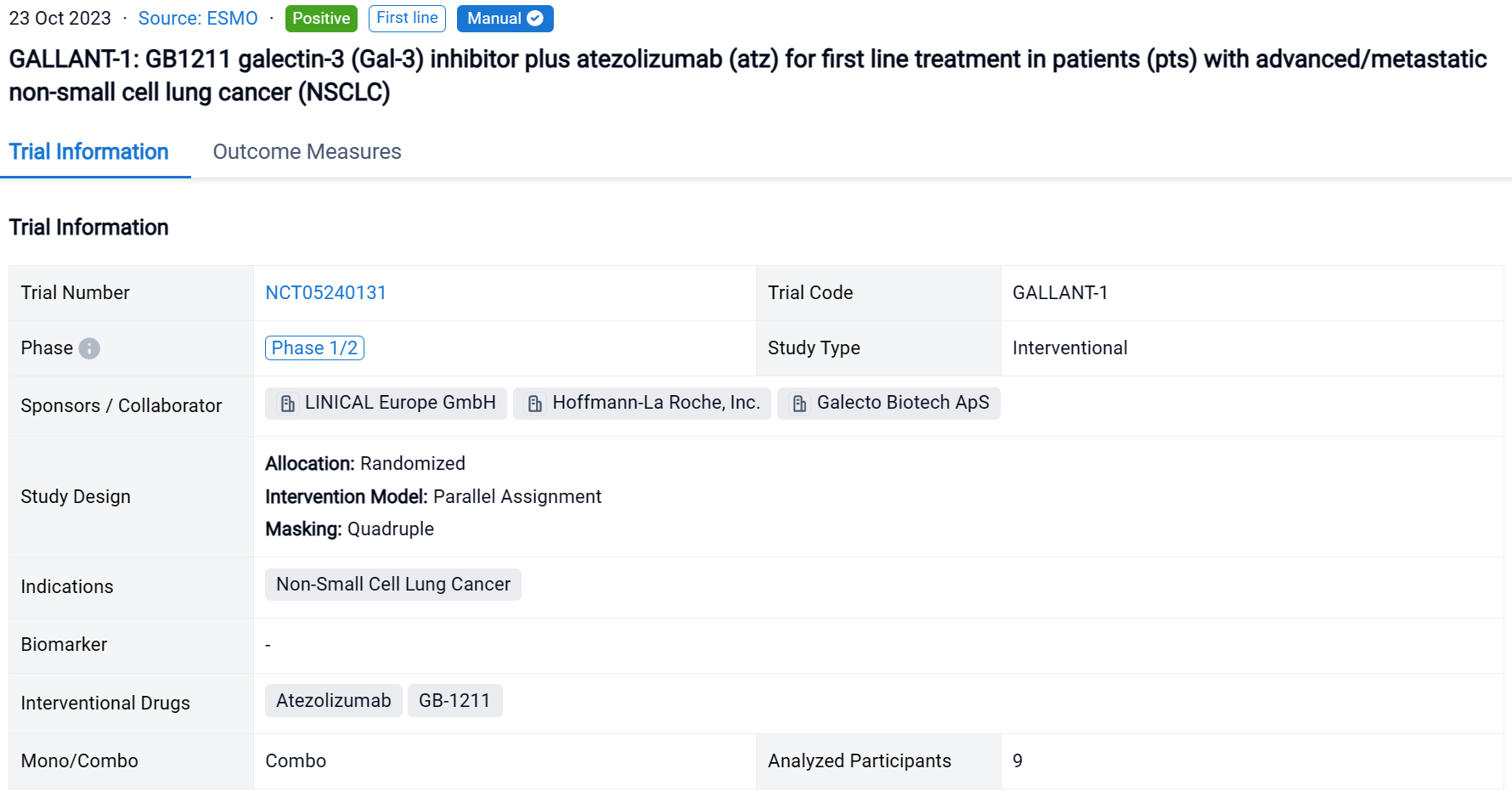
In this study, pts with advanced/metastatic NSCL were enrolled. GB1211 200 mg or 100 mg twice daily (BID) + atz 1200 mg every 3 weeks.
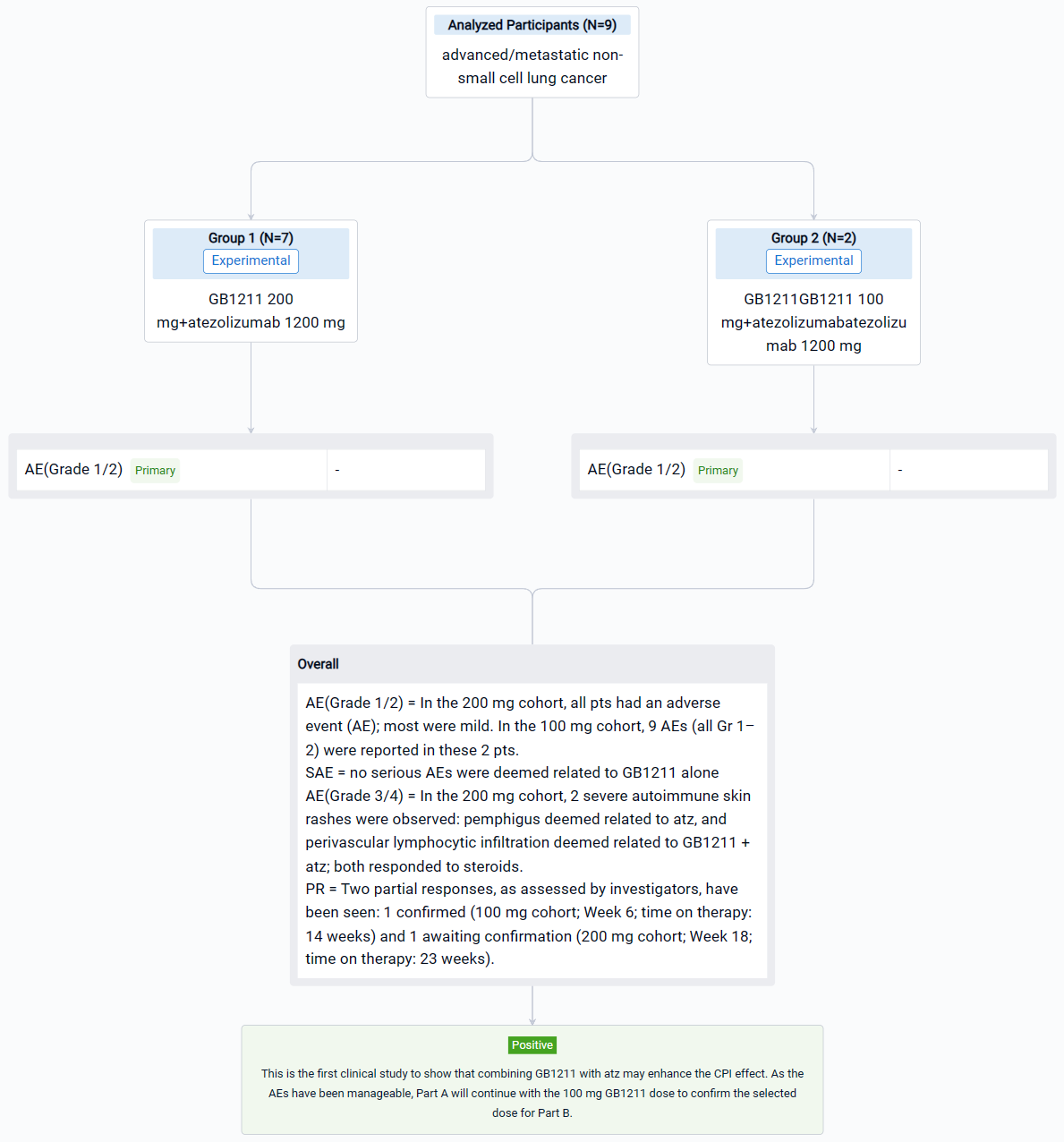
The result showed that in the 200 mg cohort (n = 7), all pts had an adverse event (AE); most were mild (Grade [Gr] 1–2) and no serious AEs were deemed related to GB1211 alone. After 2 weeks of therapy, 2 severe (Gr 3–4) autoimmune skin rashes were observed: pemphigus deemed related to atz, and perivascular lymphocytic infiltration deemed related to GB1211 + atz; both responded to steroids. As per protocol, it was recommended that the study continue but with a reduced dose (100 mg BID) in new pts (n = 2); 9 AEs (all Gr 1–2) were reported in these 2 pts. Two partial responses, as assessed by investigators, have been seen: 1 confirmed (100 mg cohort; Week 6; time on therapy: 14 weeks) and 1 awaiting confirmation (200 mg cohort; Week 18; time on therapy: 23 weeks). Steady-state plasma exposure at 200 mg was consistent with pharmacokinetic (PK) simulations, predicting a 2-fold increase compared with healthy volunteers at 100 mg. GB1211 PK did not seem altered in pts with NSCLC vs healthy volunteers.
It can be concluded that combining GB1211 with atz may enhance the CPI effect. As the AEs have been manageable, the 100 mg GB1211 dose will provide an evidence for the following study.
How to Easily View the Clinical Results Using Synapse Database?
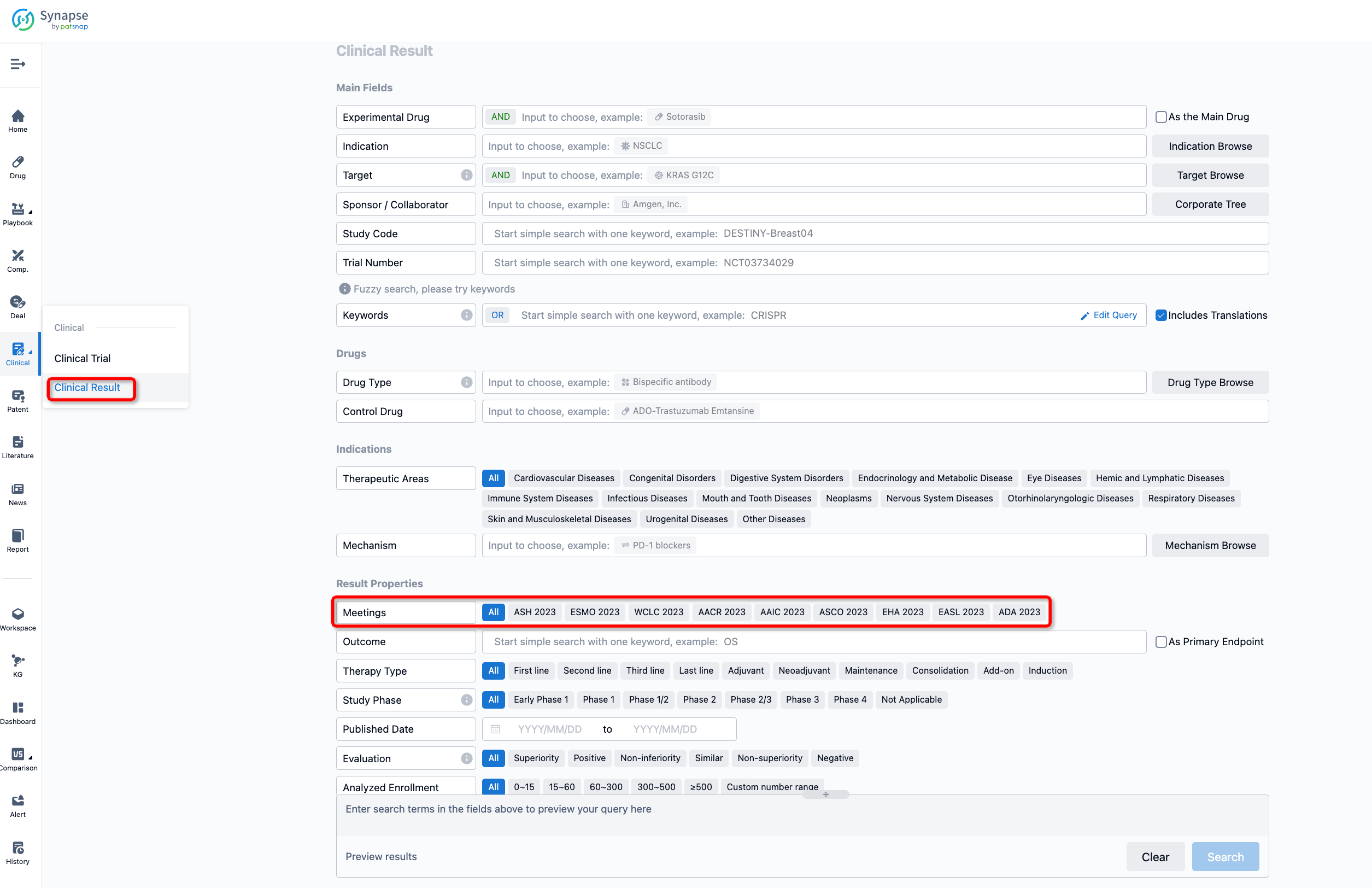
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
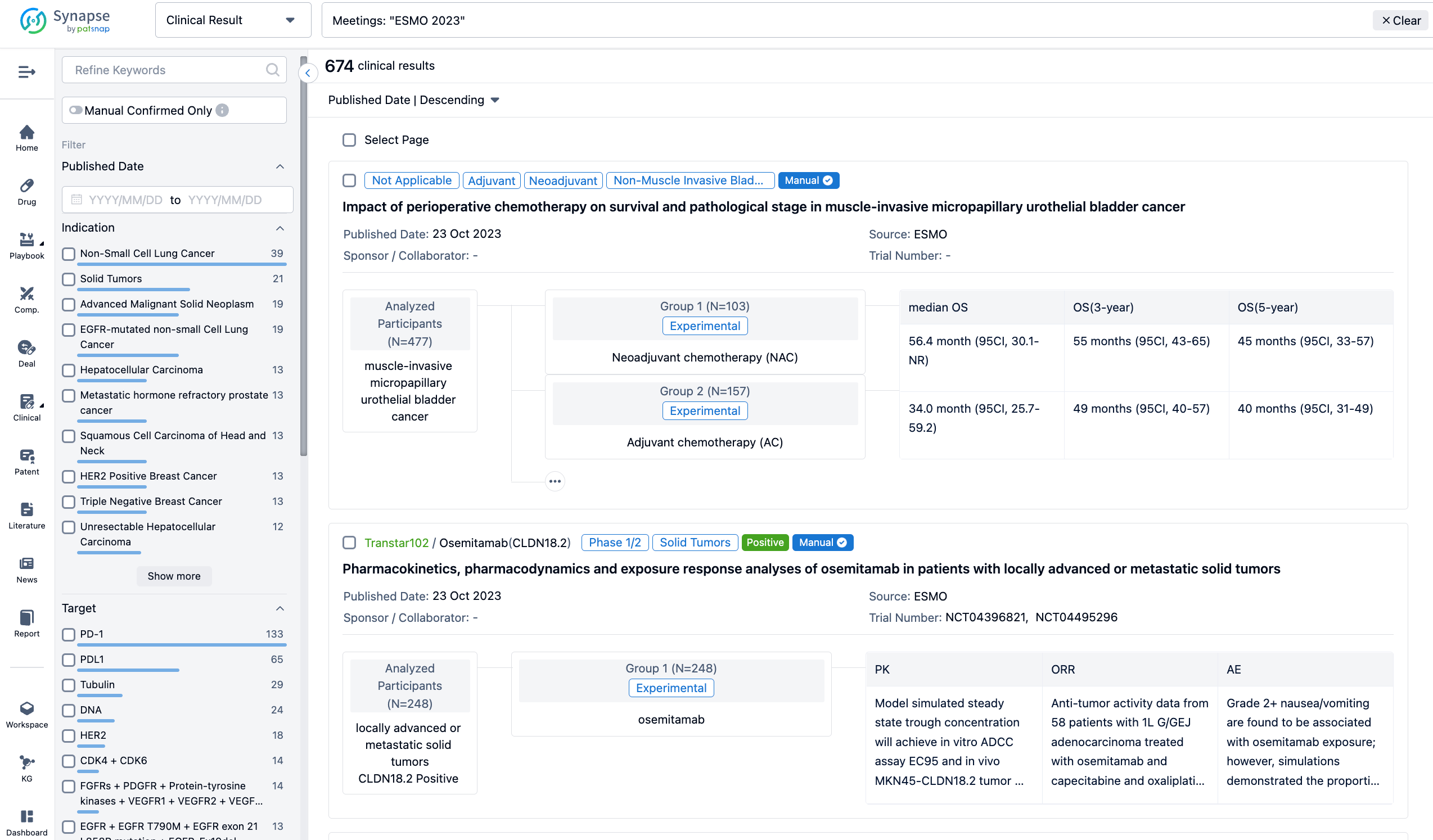
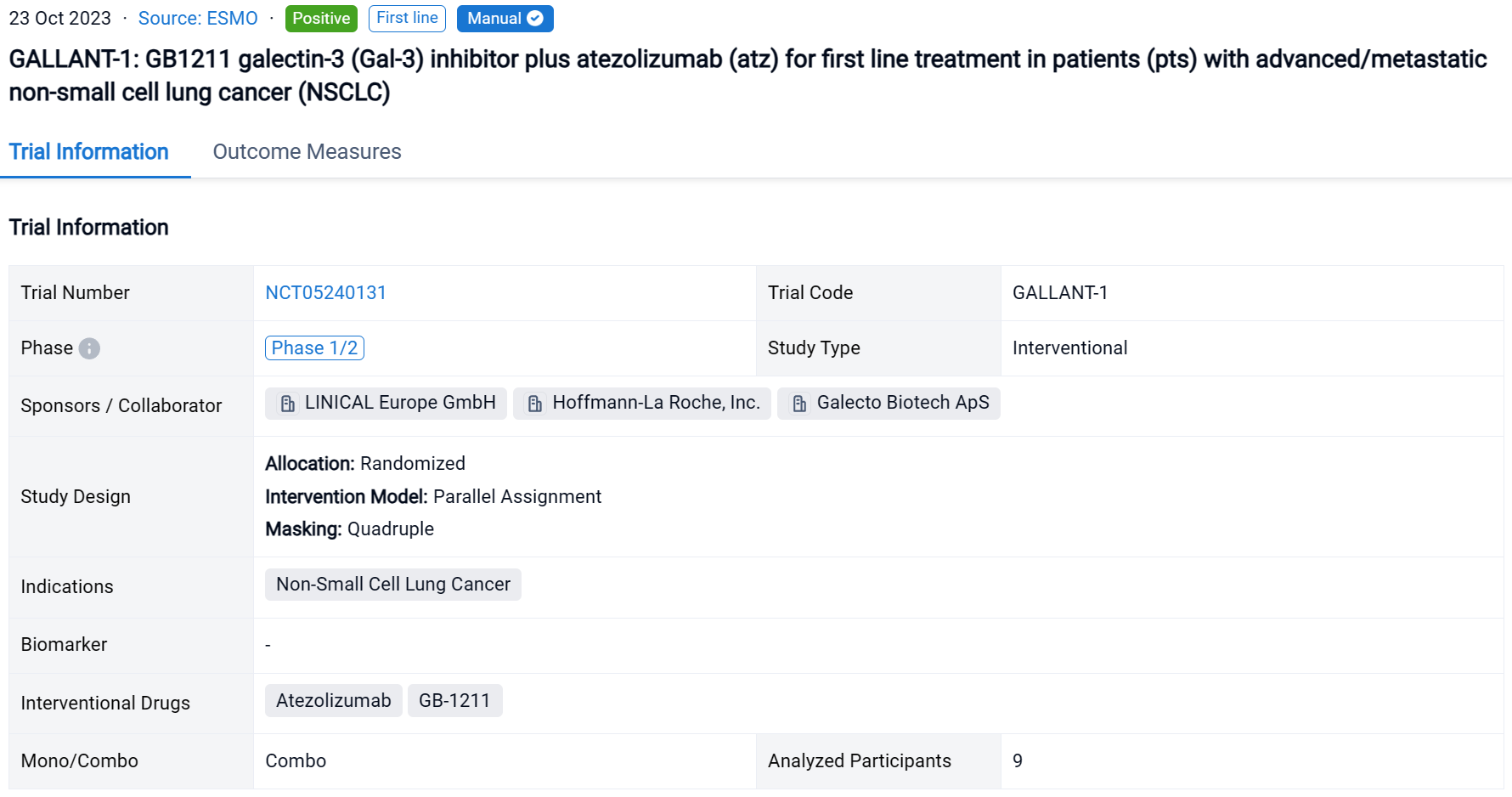
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
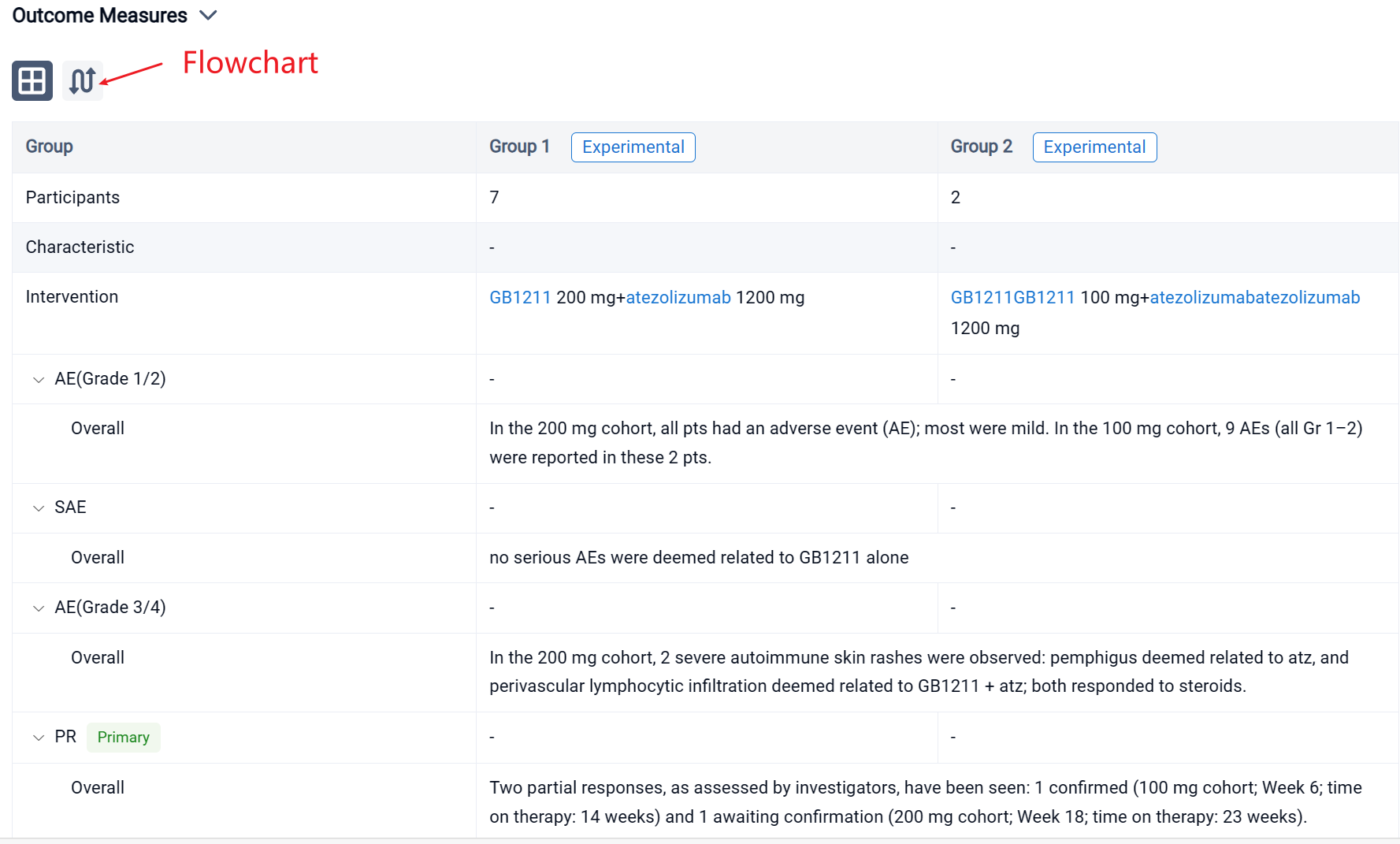
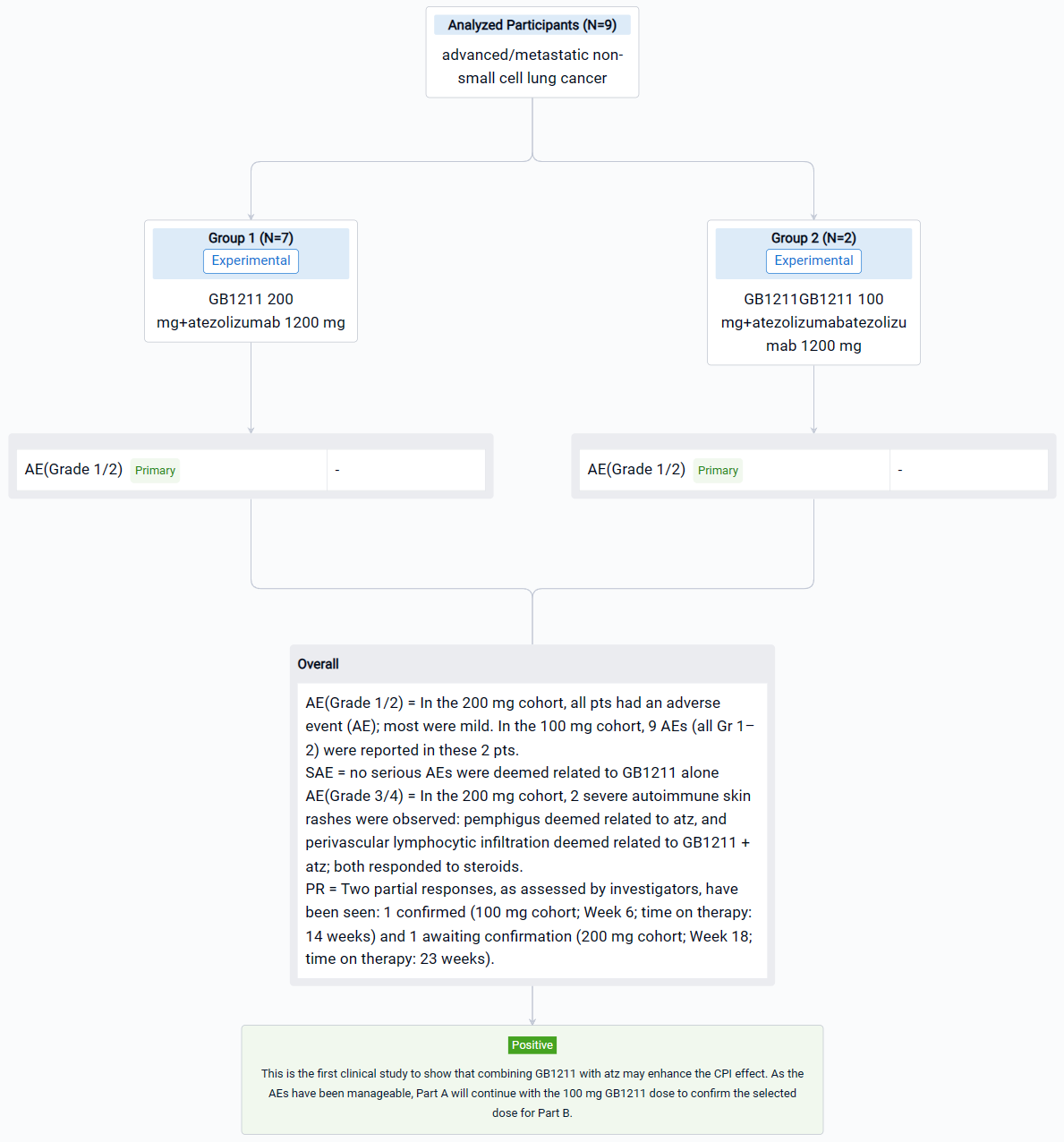
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
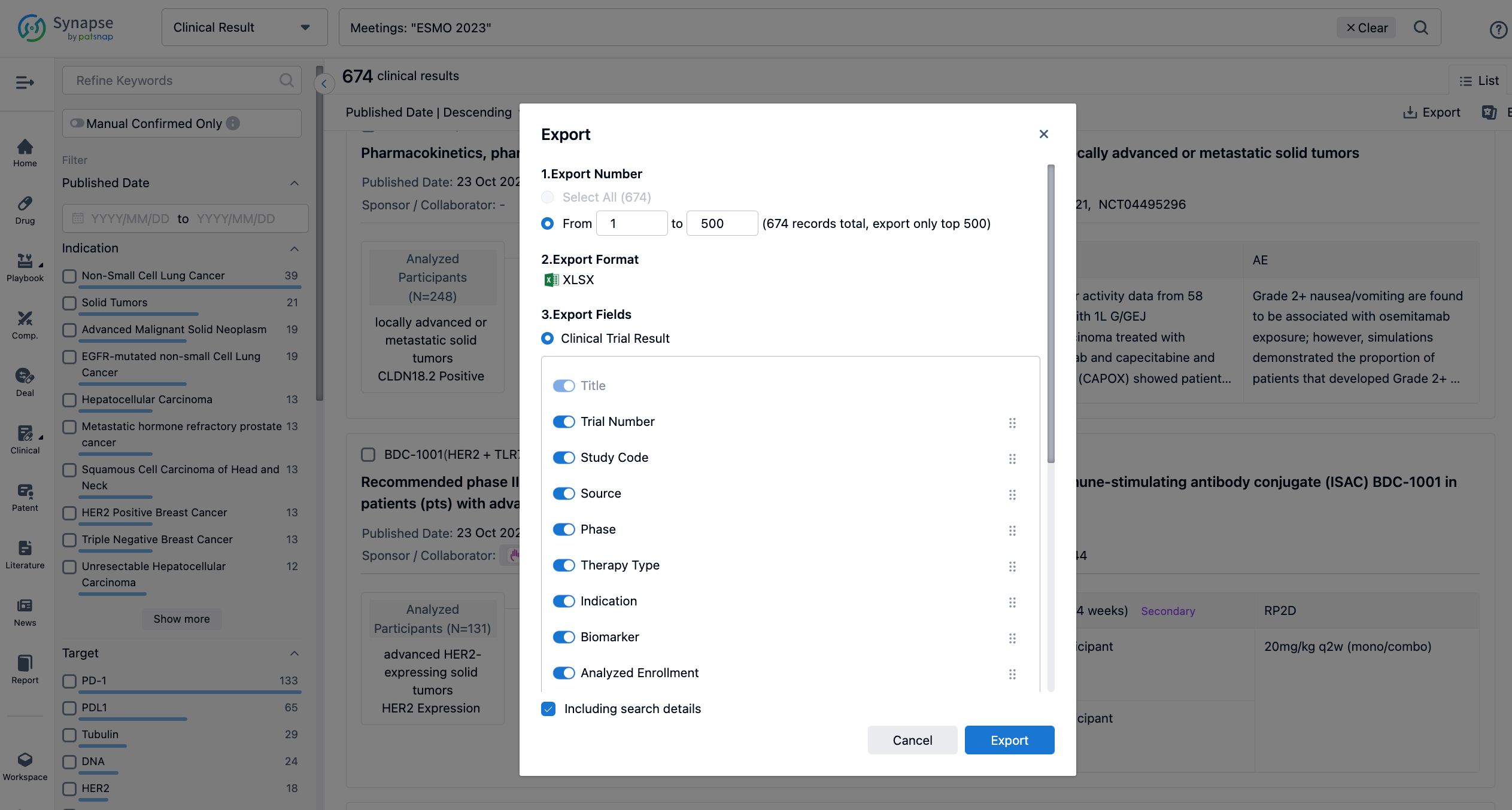
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!