GC Biopharma and Hanmi Pharmaceutical get U.S. FDA IND approval for Phase 1/2 trial
GC Biopharma revealed that its joint effort with Hanmi Pharmaceutical on the Fabry treatment 'LA-GLA' (GC1134A/HM15421) has been granted Investigational New Drug (IND) approval by the U.S. Food and Drug Administration (FDA) to initiate a Phase 1/2 clinical study.
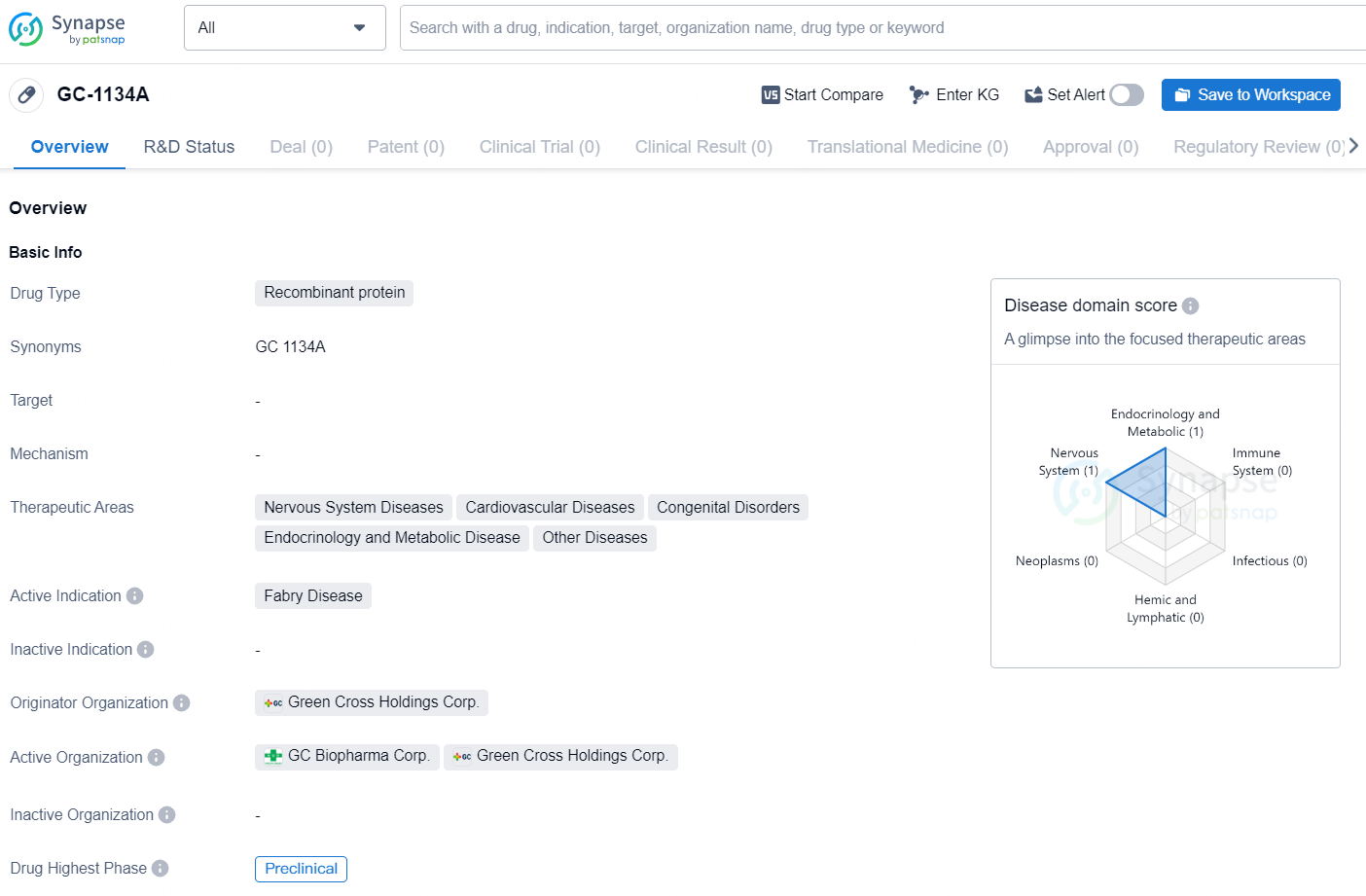
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
LA-GLA represents a groundbreaking medication for Fabry disease, standing as the world’s premier monthly subcutaneous treatment. GC Biopharma and Hanmi Pharmaceutical are jointly advancing its development. The purpose of this clinical trial is to assess the safety, tolerability, pharmacokinetics, and pharmacodynamics of LA-GLA in individuals afflicted with Fabry disease.
Fabry disease, a rare X-linked genetic disorder, is categorized under Lysosomal Storage Diseases (LSDs). It stems from a shortfall in the enzyme alpha-galactosidase A, crucial for the degradation of glycolipids within lysosomes. This enzymatic deficiency leads to the buildup of glycolipids, causing cellular toxicity and inflammatory responses, which progressively damage various organs and can result in severe health outcomes, including mortality.
Currently, Enzyme Replacement Therapy (ERT) is the common treatment for Fabry disease, which entails bi-weekly intravenous infusion of a recombinant enzyme. This method demands patients to make frequent hospital visits, posing significant inconvenience. Additionally, it is hampered by the challenges of prolonged intravenous therapy and its partial ineffectiveness in forestalling the progression of renal disease.
LA-GLA stands out as an ‘innovative enzyme replacement therapy,’ overcoming the limitations of current treatments. It offers significantly enhanced convenience through a once-monthly subcutaneous injection. Preclinical research indicated that LA-GLA not only improves renal function but also outperforms existing therapies in managing vascular disease and peripheral nerve disorders. Reflecting these promising results, it was granted Orphan Drug Designation (ODD) by the U.S. FDA this past May.
GC Biopharma and Hanmi Pharmaceutical stated, “This partnership combines the FDA's latest clinical directives with the specialized technical knowledge of both firms, facilitating a swift move into the clinical stage.” They further added, “Leveraging our expertise and experience in developing treatments for Lysosomal Storage Diseases (LSD), we are committed to providing new therapeutic options for patients suffering from Fabry disease.”
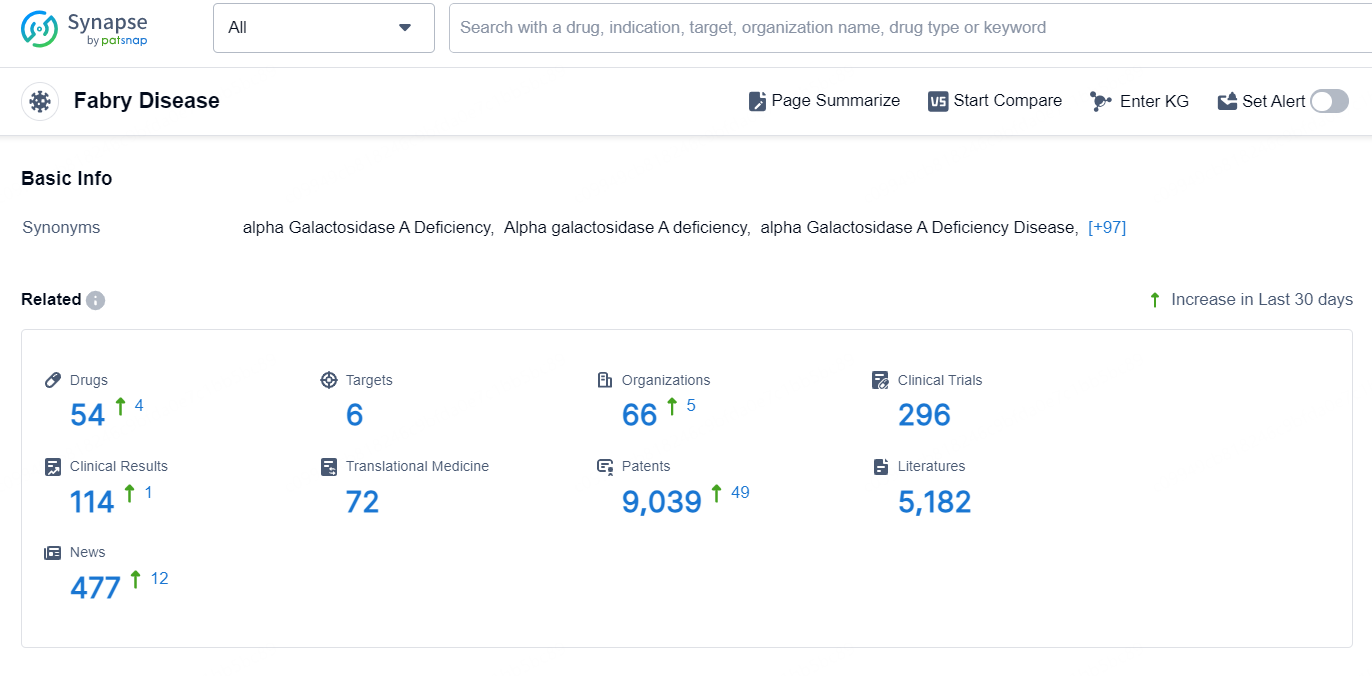
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of September 5, 2024, there are 54 investigational drugs for the Fabry Disease, including 6 targets, 66 R&D institutions involved, with related clinical trials reaching 296, and as many as 9039 patents.
GC-1134A is a recombinant protein drug developed by Green Cross Holdings Corp. It falls under the therapeutic areas of Nervous System Diseases, Cardiovascular Diseases, Congenital Disorders, Endocrinology and Metabolic Disease, and Other Diseases. The active indication for GC-1134A is Fabry Disease. As of the latest available data, GC-1134A is in the preclinical phase of development.