Genentech Reports Positive Phase Ib Results for Obesity Drug CT-388
Genentech, affiliated with the Roche Group, revealed encouraging outcomes from the Phase Ib trial of CT-388, a dual GLP-1/GIP receptor agonist aimed at addressing obesity and type 2 diabetes.
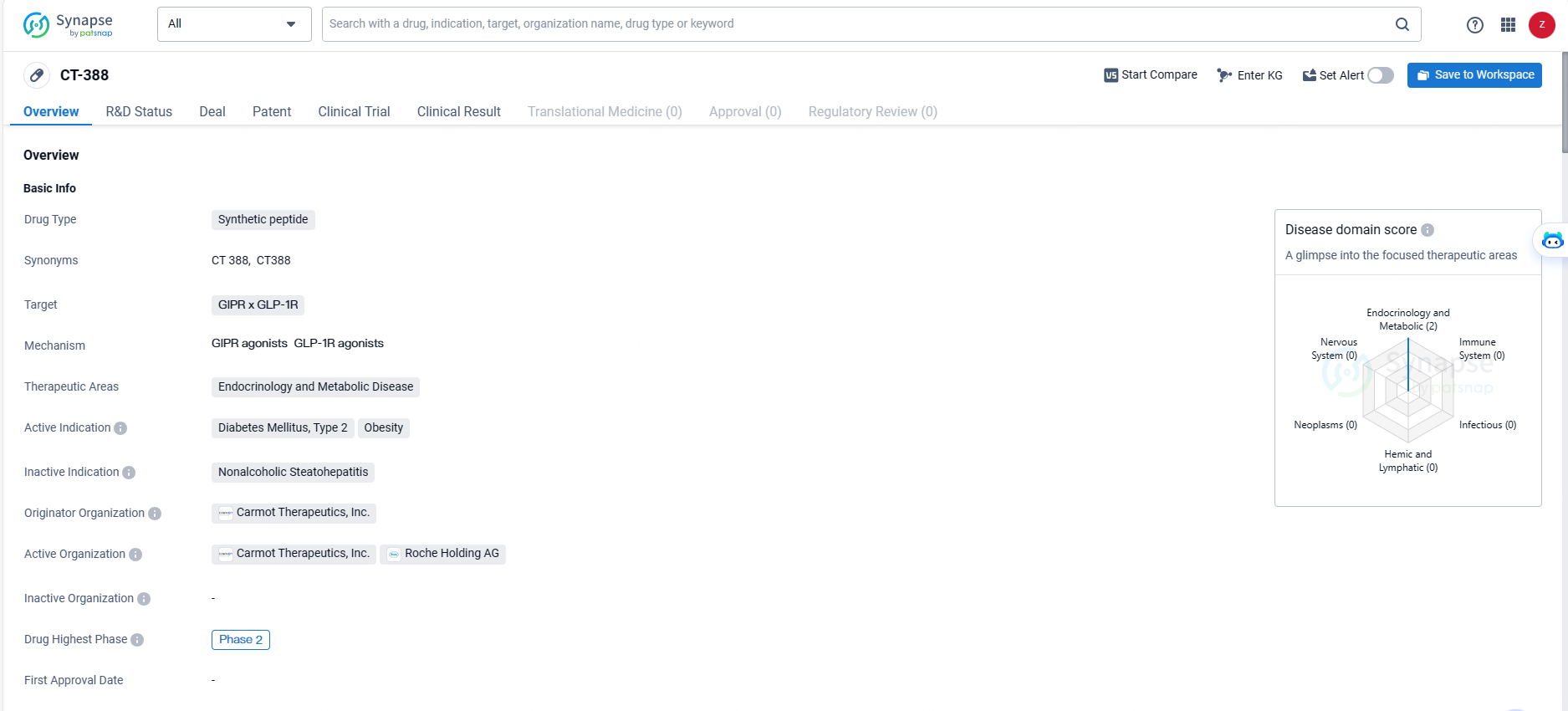
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The research discovered that a weekly subcutaneous dose of CT-388 over a period of 24 weeks led to notable weight reduction in healthy adults with obesity when compared to those given a placebo. The weight reduction achieved through CT-388 was clinically significant, showing a mean weight loss of 18.8% when adjusted for placebo. By the 24th week, all participants in the CT-388 group experienced a weight loss greater than 5%, while 85% lost more than 10%, 70% more than 15%, and 45% more than 20%.
The treatment was generally well tolerated, with the most frequent side effects being mild to moderate gastrointestinal issues, which is typical for medicines in the incretin category, to which CT-388 belongs. Additionally, all participants who had pre-diabetes at the start of the study were normoglycemic by week 24 following the CT-388 treatment, whereas the glycemic status of those on the placebo showed little to no change.
"We are extremely pleased to observe the significant and clinically relevant weight reduction in individuals treated with CT-388," commented Levi Garraway, M.D., Ph.D., chief medical officer and head of Global Product Development. "These findings are very promising for the further development of CT-388 for both obesity and type 2 diabetes, highlighting its potential to be a leading therapy with lasting benefits for weight loss and glucose regulation."
Obesity remains one of the most critical health issues globally, with numerous associated conditions such as type 2 diabetes, cardiovascular diseases, steatohepatitis, and chronic kidney disease. It is projected that by 2035, over four billion people—approximately 50% of the world’s population—will be affected by obesity or overweight, placing a massive burden on healthcare systems and societies worldwide.
CT-388 belongs to the incretin-based drug class, aiming to regulate blood sugar levels and suppress appetite. It specifically targets and activates the GLP-1 and GIP receptors in the body, which play a role in nutrient signal processing to regulate food intake, energy absorption, and assimilation. This dual targeting is hypothesized to result in significant, sustained reductions in glucose levels and body weight, alongside a favorable safety profile.
A supplementary cohort from the ongoing placebo-controlled Phase Ib trial of CT-388 will focus on obese patients with type 2 diabetes over 12 weeks of treatment. Roche anticipates data from this cohort in the latter half of 2024.
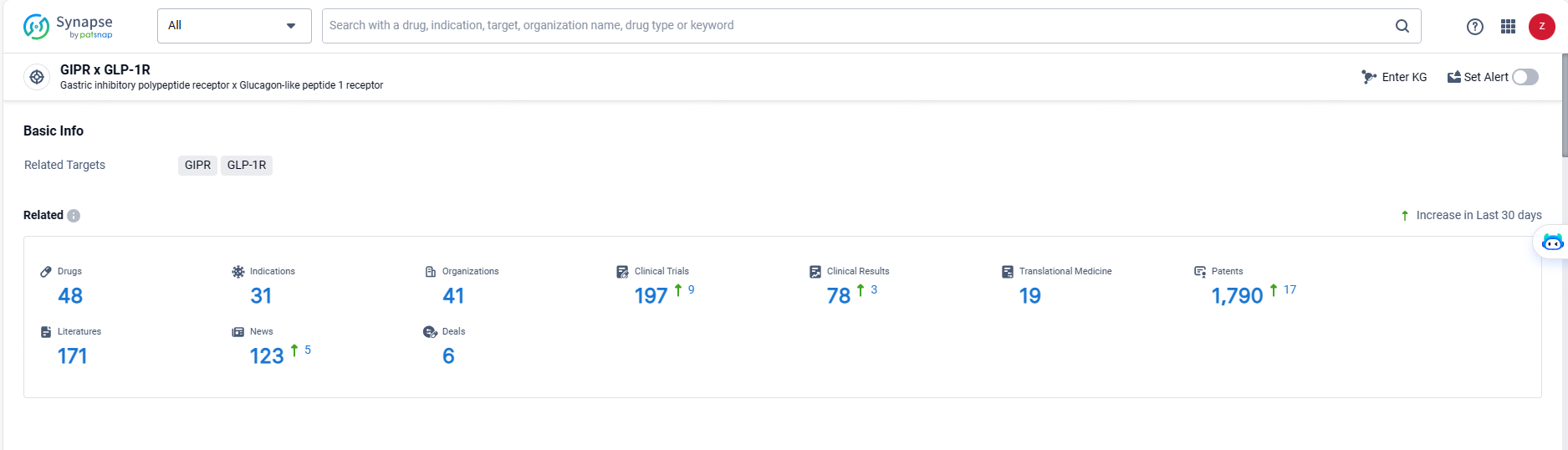
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of May 20, 2024, there are 48 investigational drugs for the GLP-1/GIP target, including 31 indications, 41 R&D institutions involved, with related clinical trials reaching 197, and as many as 1790 patents.
CT-388 is a once-weekly subcutaneous injectable, dual GLP-1/GIP receptor agonist being developed for the treatment of obesity and type 2 diabetes. The CT-388-101 trial is a multi-arm, multi-cohort Phase Ib randomized, double-blind, placebo-controlled study designed to evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics of CT-388 in otherwise-healthy adult participants with overweight or obesity and in participants with obesity and type-2 diabetes mellitus. The primary endpoint of the trial is safety and tolerability of CT-388; secondary endpoints include its effect on body weight and glucose homeostasis. Pharmacokinetics and other pharmacodynamic effects of CT-388 were also assessed.