Guangzhou Bio-gene Launches Phase I Trial for CLL-1 CAR-T Therapy BG1805
Guangzhou Bio-gene Technologies, a firm specializing in biotechnology, recently declared that it has commenced treatment on the first participant using BG1805. This experimental therapy, an autologous CAR-T cell therapy, focuses on CLL-1, which is part of the C-type lectin-like receptor family. The company is actively pursuing the research, development, and distribution of innovative cell therapies for combating blood cancers and solid tumors. BG1805 is currently undergoing a Phase 1 clinical trial aimed at addressing relapsed or refractory acute myeloid leukemia, a serious condition impacting both adults and children.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
"CLL-1 serves as a crucial target for AML therapy," Dr. Min Luo, Chief Technical Officer at Guangzhou Bio-gene Technologies, expressed his enthusiasm. "The preclinical outcomes and preliminary results from Investigator Initiated Trials regarding BG1805 have been very promising." Dr. Luo added, "BG1805 represents the initial therapy from our collection of cell therapies to move into official clinical trials, highlighting a substantial advancement for our firm."
The active Phase 1 clinical trial of BG1805 is conducted across multiple centers and is an open-label trial focusing on patients with AML who have not responded to previous treatments. The main goals of this trial are to determine its safety, its tolerability, the optimal dosage for Phase 2, and the appropriate lymphodepletion approach.
Primary safety measures of the study involve monitoring for adverse events that appear following treatment and those related to the treatment. The chief measures of effectiveness are the rate of patients experiencing full or partial remission, the percentage qualifying for hematopoietic stem cell transplantation, and those achieving a status of minimal residual disease negativity. Enrollment for the Phase 1 trial of BG1805 is currently open at several locations.
Additionally, Guangzhou Bio-gene Technologies is progressing the development of a range of innovative autologous CAR-T cell therapies, targeting critical targets in blood cancers and solid tumors, such as CD7, GPRC5D, B7H3, Claudin18.2, and CDH17. The company is also working on allogeneic CAR-γδT therapies for targets like CD19 and CLL-1/CD33.
BG1805, an innovative investigational autologous CAR-T cell therapy that focuses on CLL-1, is undergoing a Phase 1 clinical evaluation. Previous investigations into BG1805, including Investigator Initiated Studies, have shown notable effectiveness in treating both adults and children.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
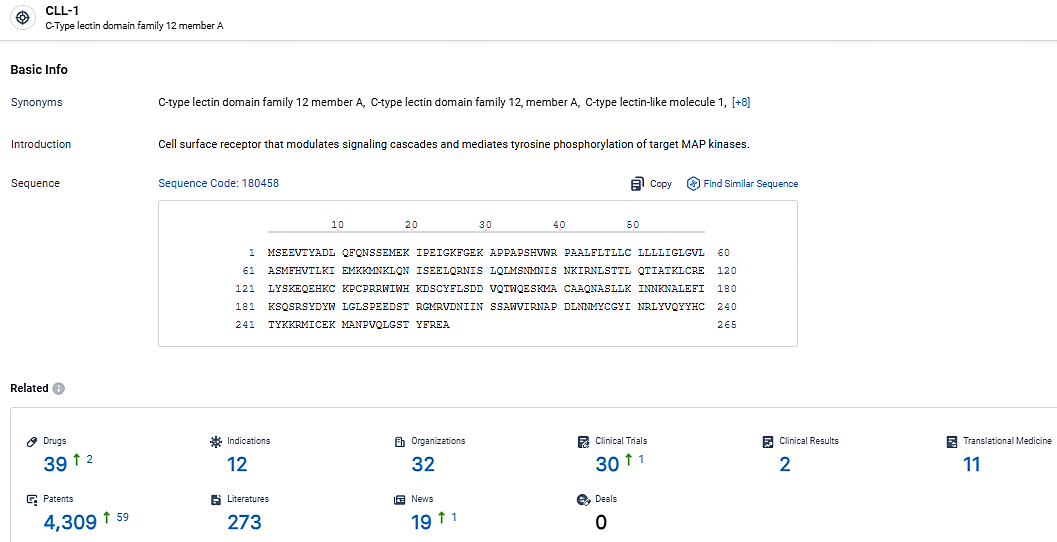
According to the data provided by the Synapse Database, As of May 6, 2024, there are 39 investigational drugs for the CLL-1 targets, including 12 indications, 32 R&D institutions involved, with related clinical trials reaching 30, and as many as 4309 patents.
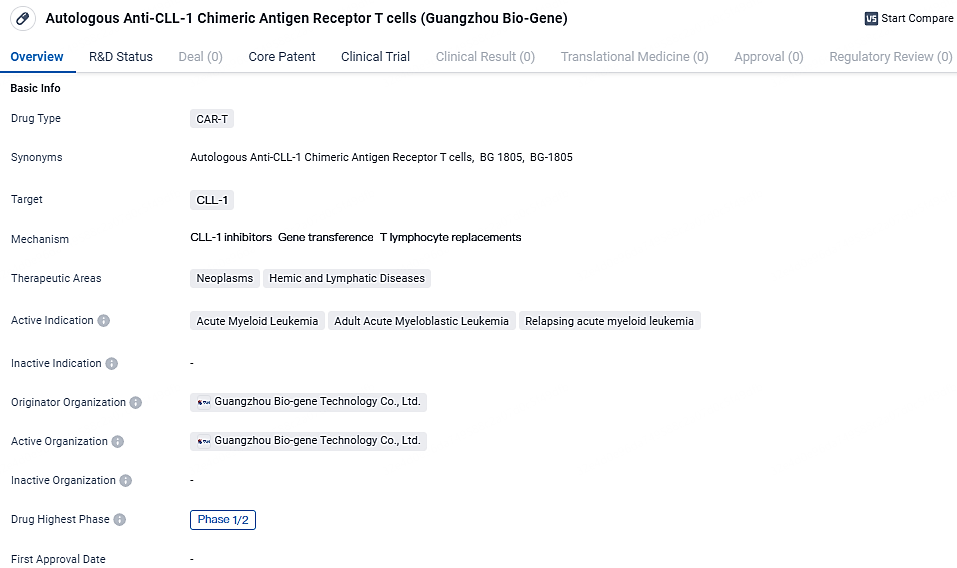
Currently, Autologous Anti-CLL-1 CAR-T cells are in the highest phase of clinical development, which is Phase 1/2, both globally and in China. Phase 1/2 trials involve testing the drug's safety and efficacy in a small group of patients to determine the appropriate dosage and evaluate any potential side effects. This indicates that the drug is still in the early stages of development and further studies are needed to assess its effectiveness in larger patient populations.