Harbor BioMed Confirms U.S. Approval to Begin Trials with HBM9027
Harbour BioMed recently disclosed that it has received authorization from the U.S. Food and Drug Administration (FDA) to begin human testing for their bispecific antibody, HBM9027, within the United States. The firm has now acquired the necessary Investigational New Drug approval to launch this initial stage of clinical trials.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
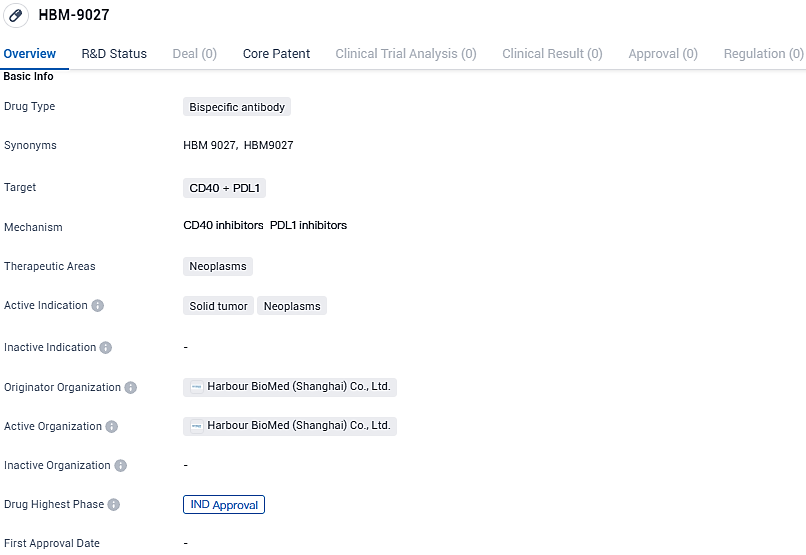
Conducting an initial clinical trial, the objective is to assess how well patients with progressing solid tumors can tolerate the novel therapeutic agent HBM9027, as well as to evaluate the drug's pharmacokinetic properties, safety measures, and effectiveness in combating cancer. HBM9027 originates from the innovative HBICE® technology owned by Harbour BioMed. This groundbreaking PD-L1xCD40 dual-specificity antibody is engineered to initiate CD40 signaling only in the presence of PD-L1 engagement, which is anticipated to offer a favorable safety profile.
Frequently found in high amounts on various solid tumor types, PD-L1, when targeted by HBM9027, links to CD40 in a manner selective to cancer cells, consequently enabling potent modulation of the immune response. According to previous laboratory investigations, HBM9027 has revealed itself to be both exceedingly safe and efficacious against cancer cells.
Emphasizing the promise of HBM9027, Dr. Jingsong Wang, the architect behind Harbour BioMed, asserts that the molecule's impressive safety track record and strong anti-cancer properties have been pivotal in showcasing the potent potential of their HBICE® platform. Dr. Jingsong Wang maintains a position of Chairman and CEO at the firm and believes wholeheartedly that Harbour BioMed's dedication to novel research will significantly enhance cancer treatments.
HBM9027 emerges as an innovative PD-L1xCD40 dual-specificity antibody, articulated through Harbour's HBICE bispecific engineering and Harbour Mice technology. The progression of PD-L1xCD40 using the HBICE approach extends the company's repertoire within the burgeoning realm of direct DC/myeloid cell engagement. Notably, the HBICE platform is reputed for its flexible geometric configurations and its capacity for seamless integration within novel therapeutic strategies.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
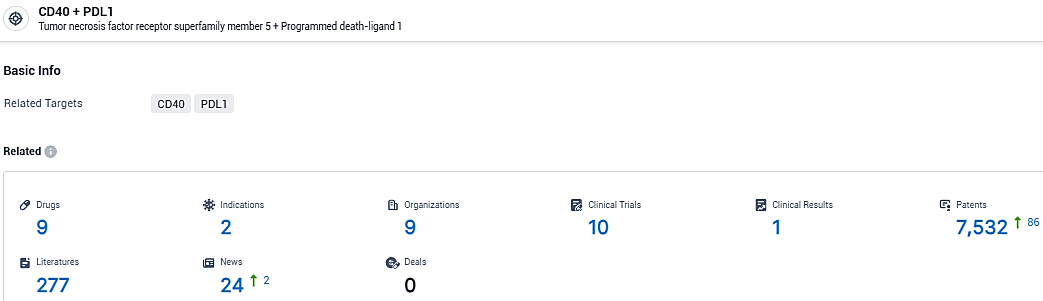
According to the data provided by the Synapse Database, As of January 29, 2024, there are 9 investigational drugs for the CD40 and PDL1 target, including 2 indications, 9 R&D institutions involved, with related clinical trials reaching 10, and as many as 7532 patents.
HBM-9027 targets CD40 and PDL1 and is intended for the treatment of neoplasms, specifically solid tumors. The drug has reached the highest phase of development, IND approval, on a global scale, indicating its potential for clinical trials in humans. However, in China, HBM-9027 is still in the preclinical stage of development.