Biosion's New Drug BSI-082 Receives FDA Approval for Investigational Application
Biosion USA, Inc., a worldwide biotech enterprise focused on clinical-stage research and development, has confirmed that its new drug proposal for BSI-082, an innovative SIRPα targeting monoclonal antibody, has received approval from the U.S. Food and Drug Administration for investigational use.
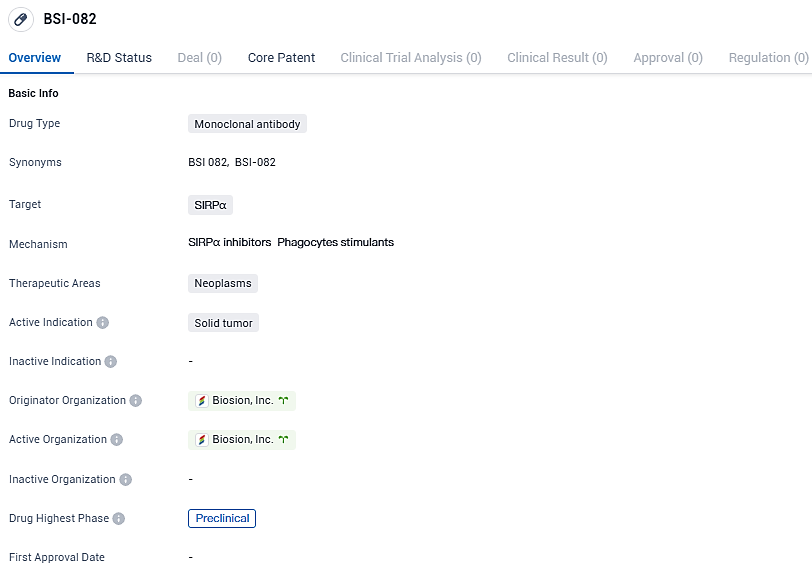
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The authorization granted by the FDA for our experimental drug, BSI-082, signifies a critical achievement for our company, Biosion, and our ongoing endeavors to invent and cultivate antibody treatments for the global patient population," expressed Mingjiu Chen, Ph.D., the originator and the CEO of Biosion, Inc.
Dr. Chen further elaborated, "The creation of BSI-082 was a result of the innovative work of Biosion's H³ antibody discovery engine, which has produced seven candidates for clinical trials within our cutting-edge pipeline. The sanctioning of this IND underlines the distinctive strengths and promise held by our proprietary antibody discovery technology."
Hugh M. Davis, Ph.D., the Chief of Business & Development as well as the President of Biosion's operations in the USA, commented, "We are gratified with the FDA's decision to permit the clinical research phase for BSI-082. This pioneering anti-SIRPa monoclonal antibody, mAb, offers multiple therapeutic possibilities for patients - it is envisioned to work in synergy with a diverse range of tumor-fighting agents including mAbs that facilitate ADCC/ADCP, immunotherapy treatments, and Antibody Drug Conjugates, to amplify the destruction of both blood and solid form tumors."
BSI-082 sets a new standard as a unique, fully human, anti-SIRPα blocking mAb. It shows substantial affinity for binding with the huSIRPα types V1/V2/V8, which allows it to potentially be applicable to over 90% of the human population. BSI-082 is selective in its interaction with SIRPα and SIRPβ, without affecting SIRPγ, effectively disrupting the SIRPα-CD47 interaction which is a common defense mechanism on a wide array of tumor cells.
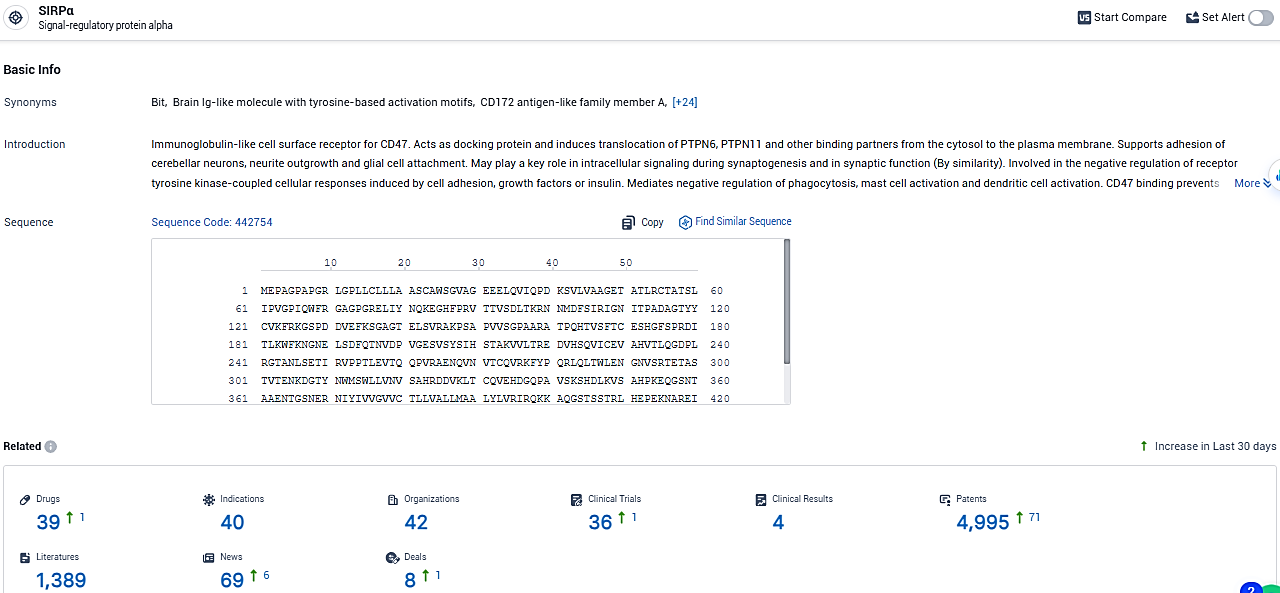
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of January 29, 2024, there are 39 investigational drugs for the SIRPα target, including 40 indications, 42 R&D institutions involved, with related clinical trials reaching 36, and as many as 4995 patents.
BSI-082 targets SIRPα and is intended for the treatment of neoplasms, specifically solid tumors. Currently, BSI-082 is in the preclinical phase globally and in China, indicating that it is still undergoing laboratory and animal testing before potential clinical trials.