Health Canada Approves Celltrion's Steqeyma® Biosimilar for Inflammatory Diseases
Celltrion Healthcare Canada Limited revealed that Steqeyma (ustekinumab injection) and Steqeyma I.V. (ustekinumab for injection, solution for intravenous infusion) have received approval from Health Canada. These approvals are for the treatment of adults with moderately to severely active Crohn’s disease, adult patients with moderate to severe plaque psoriasis, and individuals with active psoriatic arthritis.
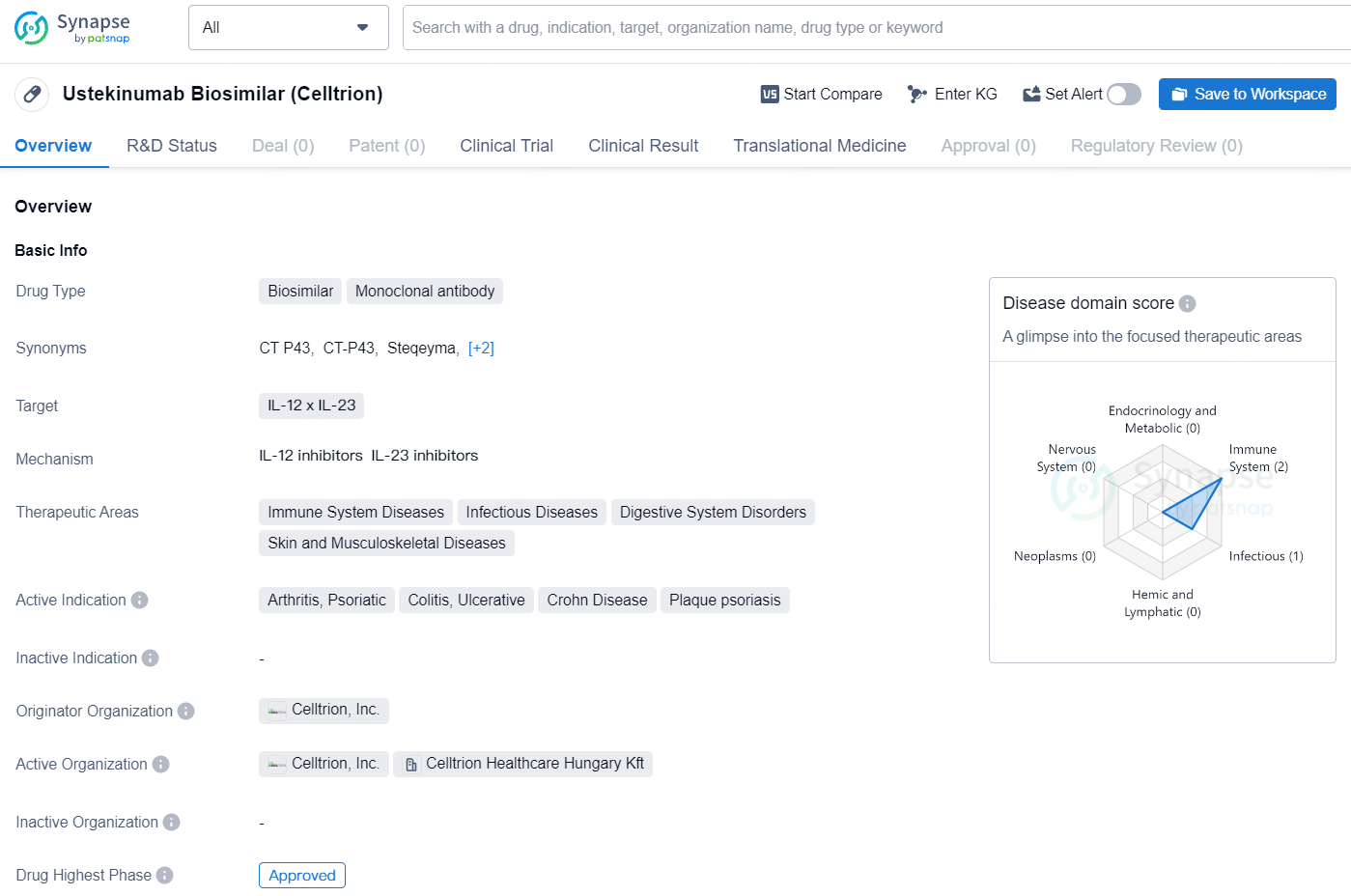
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
"We are thrilled to have Steqeyma join our development pipeline, further cementing our footprint in the field of immunology," stated Jungyong Shin, Managing Director at Celltrion Healthcare Canada. "This approval highlights our deep commitment to broadening patient access to high-quality, cost-effective biologic medications."
The approval was granted based on comprehensive evidence, including outcomes from a Phase III trial, where the main endpoint was the rate of change in the Psoriasis Area and Severity Index for skin symptoms.
The efficacy results were found to be similar between the treatment groups up to Week 52 for patients with moderate-to-severe plaque psoriasis. At Week 16, half of the patients who had initially been treated with the reference product, Stelara, switched to Steqeyma and showed comparable efficacy and safety to those who continued on the reference product. Steqeyma was well-tolerated and featured a safety profile similar to the reference product ustekinumab, with no significant safety concerns.
"Plaque psoriasis and psoriatic arthritis are severe, inflammatory conditions that significantly impact patients’ quality of life," commented Dr. Kim Alexander Papp, MD, PhD, Probity Medical Research, Waterloo, and University of Toronto. "Psoriasis affects approximately 1 million Canadians. The arrival of this new therapeutic option provides renewed optimism for those managing psoriasis and associated chronic inflammatory diseases."
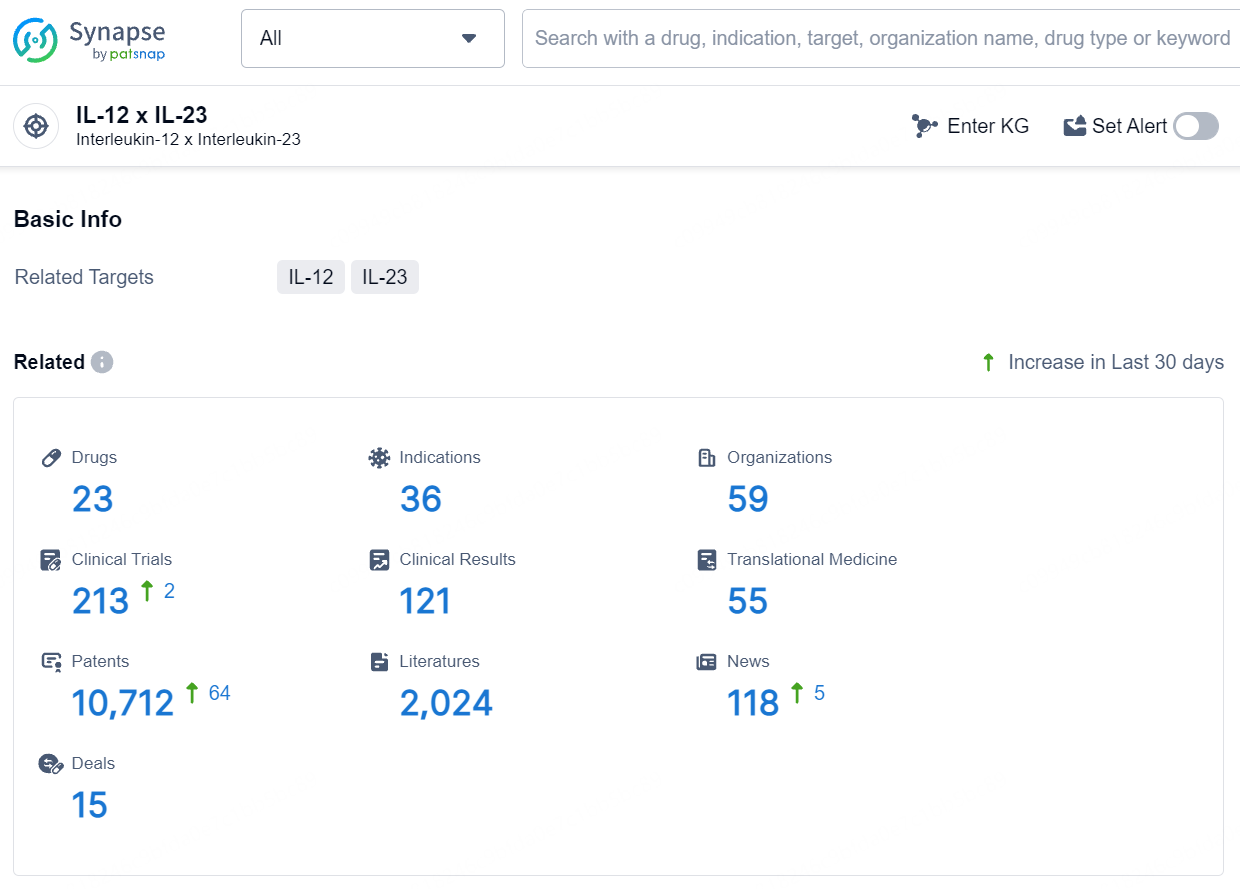
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of August 6, 2024, there are 23 investigational drugs for the IL-12 and IL-23 target, including 36 indications, 59 R&D institutions involved, with related clinical trials reaching 213, and as many as 10712 patents.
Steqeyma is a monoclonal antibody biosimilar drug that targets IL-12 x IL-23 and is indicated for various immune-related and inflammatory conditions. Its first approval was in South Korea in June 2024, and it holds potential in the treatment of arthritis, colitis, Crohn Disease, psoriasis, and other related disorders. The originator organization of this drug is Celltrion, Inc., and it has successfully passed the highest phase of approval globally.